Abstract
We have investigated the possibility that nitric oxide (NO) synthesis may affect the course of a trypanosome infection via T-cell responses using mice deficient in inducible NO synthase (iNOS). Parasitemia levels increased at the same rate in both iNOS-deficient homozygous and control heterozygous mice, and peak parasitemia values were the same in both groups. However, the heterozygous mice maintained higher parasitemia levels after the peak of an infection than the homozygous mice due to a decrease in the rate of clearance of parasites. In iNOS-deficient mice there was an increase in the numbers of total CD4+ cells and activated (interleukin-2 receptor-expressing) CD4+ cells in infected mice compared with the numbers in uninfected mice. Spleen cells from infected iNOS-deficient mice displayed increased proliferative responses and gamma interferon secretion when stimulated in vitro than those of control mice. These data suggest that NO production depresses T-helper 1-like responses generated during Trypanosoma brucei infections, thus promoting the survival of the parasite.
Trypanosoma brucei infections cause African sleeping sickness in humans and nagana in cattle. Infections are accompanied by severe immunodepression in humans, domestic animals, and experimental rodent hosts, one facet of which is a profound depression of lymphocyte responsiveness (2, 25). Immunodepression is mediated by activated “suppressor” macrophages (2, 5, 8, 13, 18, 20, 25). Lymphocyte unresponsiveness is associated with depressed interleukin-2 (IL-2) and IL-2 receptor expression (18, 20, 22) and is gamma interferon (IFN-γ) dependent (6). High levels of plasma IFN-γ occur early in infection (4), and one mechanism by which suppressor macrophages modulate lymphocyte function is via the activation products nitric oxide (NO) and prostaglandins (13, 18, 20, 24). In murine trypanosomiasis, therefore, NO causes damage to the lymphocyte function of the host. Recent studies on human and nonhuman primate infections with T. brucei suggest that NO production is elevated to levels similar to those found in the mouse infection model (26), although the functional significance in terms of immunodepression has yet to be determined.
The NO synthesized by splenic and peritoneal macrophages from trypanosome-infected mice has no trypanocidal activity (31). Although the growth of T. brucei is prevented when the organism is cocultured with activated macrophages or chemical donors of NO, trypanosomes in vivo are protected from any toxic effects as a result of their bloodstream habitat, in which hemoglobin acts as a sink for NO production (12). Inhibition of NO synthase (NOS) activity in the host with chemical inhibitors leads to reduced peak parasitemia levels (23). In T. brucei infections, the obverse of the general paradigm of NO as an antimicrobial effector is therefore observed.
Thus, there is clear evidence linking production of NO by activated macrophages to depression of cellular responses. There is also evidence linking NO production to changes in parasitemia levels without a direct action of NO on the parasites themselves. The question as to whether the depression of cellular responses causes increases in parasitemia therefore arises. Manipulation of inducible NO synthase (iNOS) activity in trypanosome-infected mice should enable this question to be addressed. Previous attempts to test the effects of NO on parasitemia and the induction of anemia used inhibitors of NOS) that suffered from a lack of specificity, as all isoforms were inhibited and administration was tolerated by mice for only limited periods (11, 23). In the present study we sought direct evidence for the role of iNOS in immunodepression and levels of T. brucei parasitemia using iNOS-deficient mice.
MATERIALS AND METHODS
Mice.
iNOS-deficient mice were generated as described previously (32). Disruption of the murine iNOS gene was achieved by homologous recombination in 129sv embryonic stem cells. The recombinant allele was passed through the germ line following the mating of the embryonic stem cell chimeras with MF-1 mice (Harlan Ltd., Oxon, United Kingdom). The homozygous and heterozygous mice thus generated were backcrossed with MF-1 mice for three generations. All the mice used were from the matings of littermates and should therefore have had a similar genetic background. Peritoneal cells from mutant mice did not produce iNOS protein following activation by IFN-γ and lipopolysaccharide in vitro as judged by Western blotting. They also did not produce detectable amounts of NO after being cultured for 48 h with IFN-γ and lipopolysaccharide. By 72 h, however, a low level of nitrite was detectable in the culture supernatant of cells from the iNOS-deficient mice. This may reflect either the accumulation of nitrite produced by constitutive NOS (14) or the induction of constitutive NOS (1, 17). iNOS-Deficient mice remained competent for other aspects of macrophage function (14). Female mutant mice and their heterozygous littermates were used when 8 to 10 weeks old. Because only mice with an outbred background were available, all assays were conducted on individual mice to take into account the potential genetic variability.
Trypanosomes.
To initiate experimental infections, groups of five adult female mice were inoculated with 104 trypanosomes/mouse via an intraperitoneal route with the cloned pleomorphic trypanosome line GUTat (Glasgow University Trypanozoon antigen type) 7.2. This line produces resolving infections in BALB/c mice in which more than 95% of the population expresses the same variable antigen type (VAT), GUTat 7.2, during the first wave of parasitemia (15). Two other VATs used in this study, ILTat (ILRAD Trypanozoon antigen type) 1.3 and 1.61, are not expressed during the first 15 days of infection of the GUTat 7.2 line. In rabbits (which are less susceptible to infection than mice) these two VATs are detected at approximately days 20 through 30 of infection (15, 27a). The parasitemia of each mouse was monitored daily by removing 2 μl of blood and diluting it in 0.85% NH4Cl in phosphate-buffered saline (PBS), and parasites were counted by using an Improved Neubauer hemocytometer.
To obtain parasites for ex vivo proliferation and cytokine assays, cloned trypanosome lines that stably express GUTat 7.2, ILTat 1.3, or ILTat 1.61 were grown in adult female CFLP mice (Harlan Olac) and purified from infected mouse blood (9). Trypanosomes were suspended in 0.1 M PBS (pH 7.4) and counted. They were then incubated at room temperature for 20 min in 4% paraformaldehyde in PBS, washed three times in PBS by centrifugation at 900 × g for 5 min each time, and left overnight in 0.1 M NH4Cl in PBS to neutralize NH2 groups. The trypanosomes were centrifuged, resuspended in PBS at a concentration of 107/ml, and stored at 4°C for up to 4 weeks. Populations were checked for VAT homogeneity by immunofluorescence on air-dried smears fixed in 70% ethanol by using VAT-specific antibodies as previously described (29).
Isolation of mononuclear splenocytes.
Spleens were removed from individual mice, and mononuclear splenocytes were isolated. Briefly, spleens were disrupted through sterile tea strainers into RPMI 1640 medium and passed through 100-μm-pore-size monofilament nylon filters to obtain single cell suspensions. Cymelarsan (Rhone-Merieux, Lyon, France) was added to each suspension (including controls) at 50 μg/ml to kill contaminating trypanosomes, and suspensions from infected mice were monitored by microscopy until lysis of trypanosomes was observed (usually 10 to 30 min). The splenocytes were centrifuged, and loose pellets were resuspended, layered onto cushions of Nycoprep (Nycomed Ltd.), and centrifuged for 15 min at 700 × g. The mononuclear interface layer was removed and centrifuged, and the pellet was resuspended in 2 ml of medium. The cells were counted, their viability was checked by trypan blue exclusion, and the cells were resuspended at the required density. The centrifugation steps after Cymelarsan treatment removed dead parasites from these preparations, as judged by phase-contrast microscopy.
Flow cytometry analyses.
From each spleen, aliquots of 106 mononuclear splenocytes were made, resuspended in PBS with 5% fetal calf serum and 0.05% azide in 50-μl volumes, and double-labelled with phycoerythrin (PE)-conjugated anti-CD4 plus fluorescein isothiocyanate (FITC)-conjugated anti-CD8, PE-conjugated anti-CD4 plus FITC-conjugated anti-CD25α chain, or PE-conjugated anti-CD8 plus FITC-conjugated anti-CD25α chain (Pharmingen) at the manufacturer’s recommended concentrations for 1 h on ice. The cells were washed twice in PBS-fetal calf serum and resuspended in 0.1 M Tris–0.9% NH4Cl for 10 min to lyse any contaminating erythrocytes. The cells were pelleted by brief centrifugation, resuspended in PBS (pH 7.0), and analyzed with a FACScan flow cytometer (Becton Dickinson).
Proliferation assays.
The isolated mononuclear splenocytes were plated out at 2 × 106 cells/well in 200-μl aliquots in flat-bottomed 96-well microtiter plates with either medium alone, concanavalin A (ConA) (8 μg/well; Sigma), or paraformaldehyde-fixed trypanosomes (2 × 106/ml) expressing GUTat 7.2, ILTat 1.3, or ILTat 1.61. The cells were incubated at 37°C in 5% CO2 for 48 h and radiolabelled with 1 μCi of [3H]thymidine per well for the last 16 h before being harvested; results were read on a Betaplate counter. The proliferative responses were determined by [3H]thymidine incorporation, and results are expressed as the mean counts per minute (± 2 standard errors [SE]) of four wells.
Cytokine assays.
The cells were dispensed into 24-well plates at 4 × 106 cells/well in 1.25-ml volumes and incubated with either medium alone, ConA (8 μg/well), or paraformaldehyde-fixed trypanosomes (2 × 106/ml) expressing GUTat 7.2, ILTat 1.3, or ILTat 1.61. The cells were incubated at 37°C in 5% CO2, supernatants were harvested at 72 h and microcentrifuged for 30 s to remove cells, and the cell-free supernatants were stored at −20°C until analysis by enzyme-linked immunosorbent assay for IFN-γ, IL-2, and IL-5 with commercial capture and detection antibodies (Pharmingen).
Plasma nitrate levels.
The levels of nitrite and nitrate present in the plasma from individual mice were measured by the reduction of nitrate to nitrite and by the Griess reaction as previously described (7, 12).
Statistical analyses.
Parasitemia data were analyzed by repeated measures analysis of variance with log-transformed data, and levels of IFN-γ and plasma nitrate were analyzed by Mann-Whitney U tests.
RESULTS
Each experiment was conducted at least twice and produced the same pattern of data on each occasion.
Comparison of parasitemia levels in iNOS-deficient and control mice.
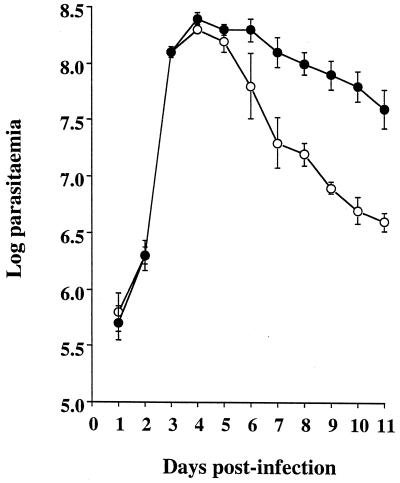
Levels of GUTat 7.2 parasitemia in iNOS-deficient and control mice increased exponentially at similar rates and reached similar densities at the peak of parasitemia on the same day postinfection (Fig. 1). Parasitemic decline after the parasitemic peak was different between the two groups (F1, 6 = 43.0, P < 0.001), however, with parasitemias in the iNOS-deficient mice decreasing more rapidly. From days 8 to 11 of infection there was an approximately 1-order-of-magnitude difference in levels of parasitemia between iNOS-deficient and control mice. These levels of parasitemia in the heterozygous MF-1 mice were higher than those previously described for BALB/c mice (30); therefore, these experiments were terminated on day 11 of infection, and several components of the immune system were assayed.
FIG. 1.
Time course of T. brucei parasitemia in iNOS-deficient (○) and heterozygous control (●) mice inoculated with 104 GUTat 7.2 parasites. Results are expressed as geometric means ±2 SE for groups of five mice.
Cytometric analyses of mononuclear splenocytes.
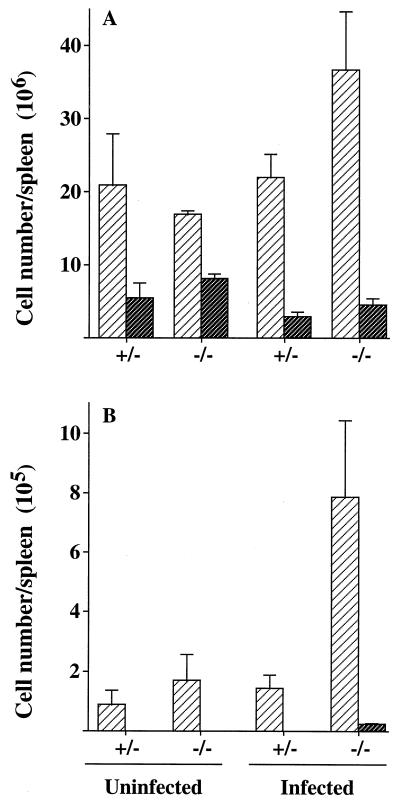
Expressing cytometric data in percentage terms fails to take into account the gross splenomegaly caused by trypanosomiasis; for this reason, our data are expressed as cell numbers per spleen. As a result of infection, CD4+-cell numbers increased in iNOS-deficient mice, and CD8+-cell numbers decreased slightly in both groups (Fig. 2). These changes caused the ratios of CD4+ cells to CD8+ cells to change from 2:1 to 8:1 in iNOS-deficient mice and from 3.8:1 to 7.3:1 in control mice. Figure 2 also shows that there were approximately fourfold more activated CD4+ cells (expressing CD25) present in the spleens from iNOS-deficient infected mice than in spleens from infected control or uninfected mice. Activated CD8+ cells were detected only in mononuclear splenocyte populations (in low numbers, 2 × 104/spleen) from infected iNOS-deficient mice.
FIG. 2.
Numbers of CD4+ (light) or CD8+ (dark) cells per spleen (A) and numbers of activated CD4+ (light) or CD8+ (dark) cells per spleen, with CD25 as a marker for cell activation (B). Numbers were determined by flow cytometry of mononuclear splenocytes of iNOS-deficient (−/−) and control (+/−) mice, either uninfected or infected with GUTat 7.2 (results are for day 11 of infection). Mononuclear splenocytes were counted, the lymphocyte population was gated and checked to exclude autofluorescence, and relative numbers were determined. From these data, cell numbers per spleen were calculated and are expressed as geometric means ±2 SE for groups of five mice.
Proliferative responses.
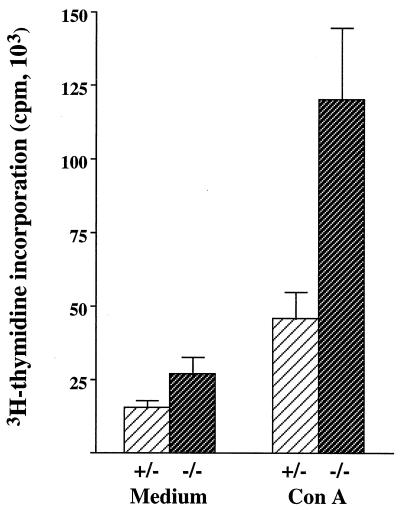
The ability of the T cells to proliferate in response to ConA was examined (Fig. 3). High levels of proliferation were observed in splenocytes from infected mice in the absence of any stimulant, which is in accord with our previous observations, and we attribute these responses to increased numbers of activated cells in infected mice (5). Nevertheless, enhanced proliferation in response to ConA was seen in control mice (2.9-fold higher) and, most notably, in the iNOS-deficient mice (4.5-fold higher) (Fig. 3). Trypanosome-specific proliferation in response to paraformaldehyde-fixed parasites expressing one of the three VATs was not observed (data not shown). We attribute the difference in ConA-induced proliferative responses between iNOS-deficient and control mice to the way in which they respond to infection rather than to any intrinsic difference between the mice. No such differences were found between uninfected iNOS-deficient and control mice with an MF-1 genetic background in a previous study (14). Also, negligible differences were found in a study of iNOS-knockout mice with a C57BL/6 genetic background (10).
FIG. 3.
Proliferative responses of mononuclear splenocytes from trypanosome-infected iNOS-deficient (−/−) (dark) and control (+/−) (light) mice harboring infections of GUTat 7.2. Splenocytes were cultured in the presence of either medium or ConA (8 μg/ml). Proliferative responses of mononuclear splenocytes from uninfected mice were <2,000 cpm in the absence of stimulation. Results are expressed as the geometric means ±2 SE for groups of five mice.
Cytokine production.
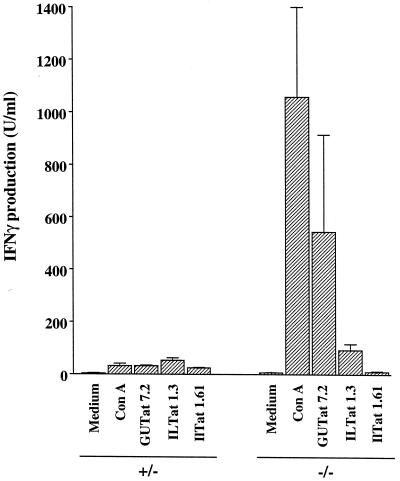
Mononuclear splenocytes from chronically infected control and iNOS-deficient mice differed in the capacity to produce IFN-γ (Fig. 4). The control mice produced very little IFN-γ when stimulated with ConA or with any of the three paraformaldehyde-fixed VATs. However, IFN-γ production by mononuclear splenocytes from the iNOS-deficient mice was very different, with high levels produced in response to ConA (W = 15, P < 0.05) and trypanosomes expressing the homologous, but not heterologous, VAT (W = 15, P < 0.05). The heterologous VATs, ILTat 1.3 and 1.61, were both undetectable by immunofluorescence analysis on days 7 and 11 of infection (data not shown), indicating that splenocytes had no detectable exposure to these VATs in vivo. The three trypanosome lines used to stimulate the mononuclear cells are of the same genetic origin and thus differ antigenically only in their VAT expression. All antigens except the variant surface glycoproteins (VSGs) (the invariant antigens) were thus the same in all three paraformaldehyde-fixed preparations. If invariant antigens were strongly immunogenic in this assay, then equivalent levels of IFN-γ production would be expected, irrespective of which antigen preparation was used as a stimulant. A similar result would also be expected if the three VSGs shared epitopes. The data shown in Fig. 4 thus confirm that VSG is the main immunogen of T. brucei and indicate VAT-specific stimulation of IFN-γ production.
FIG. 4.
IFN-γ production by mononuclear splenocytes from iNOS-deficient mice and their heterozygous counterparts harboring infections with GUTat 7.2. IFN-γ concentrations were assayed by enzyme-linked immunosorbent assay. Cells were stimulated with medium, ConA (8 μg/ml), or paraformaldehyde-fixed trypanosomes (2 × 106/ml) expressing the homologous VAT or one of the two heterologous VATs. Supernatants were harvested 72 h after stimulation. Results are expressed as means ±2 SE for groups of five mice.
In both control and iNOS-deficient infected mice, IL-5 production was <1 U/ml when stimulated with medium, ConA, or any of the three VATs (data not shown). The only detectable IL-2 production was from cells stimulated with ConA. These levels, in both groups of mice, were <10 U/ml 24 h after stimulation in vitro (data not shown).
Plasma nitrite and nitrate.
In control and iNOS-deficient uninfected mice, the mean concentrations of nitrate (±2 SE) were 24.7 ± 4.1 and 18.8 ± 2.9 μM, respectively. In the infected control mice there was a 6.7-fold increase in plasma nitrate levels, to 165.0 ± 45.3 μM (W = 49, P = 0.023), and a 3.8-fold increase in the iNOS-deficient infected mice, to 70.8 ± 7.9 μM (W = 56, P = 0.011).
DISCUSSION
Abrogation of iNOS activity had a marked affect on all indices of T-cell competence. Numbers of splenic CD4+ cells and activated CD4+ cells expressing the IL-2 receptor increased as a result of infection in the iNOS-deficient mice, whereas CD8+ cell numbers decreased and CD25 CD8 double-positive cells occurred only rarely. These data are consistent with previous observations that the T-cell response in T. brucei infection is mainly CD4 mediated (19) rather than CD8 mediated (3, 16). These data are also in agreement with the observation of a proliferative response to ConA and of the ability to generate IFN-γ, but not IL-5, in response to both ConA and specific antigen, indicating that there is a depression of T-cell responses (2, 5, 6, 8, 13, 18, 20, 22, 25) caused by NO and that responsiveness is restored on removal of NO (13, 18, 23, 24). The extent of the recovery of T-cell competence in the iNOS-deficient mice was sufficient to cause a change in parasitemia but has not been determined directly. A prediction would be that the recovery was incomplete, given that we observed raised levels of plasma nitrate in the iNOS-deficient mice and as a result of continued immunodepressive prostaglandin synthesis (18, 21). This prediction is consistent with our observation of trypanosome VAT-specific responsiveness detected by the IFN-γ assay but proliferative responsiveness detected only with mitogen. IFN-γ production in the absence of a proliferative response has been previously observed in peritoneal T cells from T. brucei-infected mice (19). Our results suggest that on day 11 of infection in this mouse model there was a generalized depression of T-cell responses, but a deficiency in NO production restored some cellular competence. The observation that the numbers of splenic CD4+ cells and activated CD4+ cells increased in iNOS-deficient mice, together with the detection of IFN-γ, but not IL-5, production from splenocytes, suggests that the cellular response is T-helper 1-like.
Our parasitology data contrast with the results from studies using NOS inhibitors, which showed a reduction in peak levels of parasitemia but no difference in rates of parasite clearance (23). This discrepancy may be due to the use of different mouse and parasite strains but also may reflect levels and sources of NO. NG-nitro-l-arginine methyl ester (l-NAME) causes a quantitative reduction in NO production by all isoforms of NOS, whereas in the present study iNOS activity was abrogated but other isoforms were unaffected. We have some evidence for compensatory production of NO from a source other than iNOS in the elevated levels of plasma nitrate in the infected iNOS-deficient mice. A low level of production of NO has been observed previously in culture supernatants of peritoneal cells from these iNOS-deficient mice (14). These elevated levels of nitrate may reflect the induction of constitutive isoforms of NOS, as has been demonstrated under other circumstances (1, 17).
It has been suggested that while NO is a mediator of immunodepression in mice, it is not so in cattle (27). However, most of the murine studies have been conducted with T. brucei, whereas the cattle study was based on Trypanosoma congolense, and these two species differ in an important aspect of their biology. T. congolense parasites adhere to the vascular endothelium whereas T. brucei does not. Macrophage activation happens mainly in the spleen and liver, but the rate of movement of trypanosomes through these organs is expected to be considerably lower for T. congolense. Thus, the quantitative extent, and perhaps qualitative nature, of macrophage activation would be expected to be very different for the two species. Studies on T. congolense-infected iNOS-deficient mice are clearly warranted.
Our results suggest that as a result of NO production, parasitemia levels declined more slowly after the first peak and indices of cellular responsiveness were lower. Establishing this link between depression and a higher level of parasitemia after the first peak is important because it has been postulated that the generation of immunodepression is an immune evasion strategy (2). This postulate can only be true, however, if depression is of selective advantage to the parasite (in evolutionary terms) rather than merely disadvantageous to the host (28). It is only by increasing the level of parasitemia, and thus promoting transmission of parasites from mammals to the tsetse fly, that a selective advantage can be obtained. The implication from our data is that trypanosomes contain genetically determined mechanisms for causing immunodepression.
ACKNOWLEDGMENTS
We thank the UK Medical Research Council and The Wellcome Trust for financial support.
We thank John Mansfield for sharing unpublished data. Cymelarsan was a kind gift from Rhone-Merieux.
REFERENCES
- 1.Amin A R, Di Cesare P E, Vyas P, Attur M, Tzeng E, Billiar T R, Stuchin S A, Abramson S B. The expression and regulation of nitric oxide synthase in human osteoarthritis-affected chondrocytes: evidence for up-regulated neuronal nitric oxide synthase. J Exp Med. 1995;182:2097–2102. doi: 10.1084/jem.182.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askonas B A. Macrophages as mediators of immunosuppression in murine African trypanosomiasis. Curr Top Microbiol Immunol. 1985;117:119–127. doi: 10.1007/978-3-642-70538-0_6. [DOI] [PubMed] [Google Scholar]
- 3.Bakhiet M, Olsson T, van der Meide P, Kristensson K. Depletion of CD8+ T cells suppresses growth of Trypanosoma brucei brucei and interferon-gamma production in infected rats. Clin Exp Immunol. 1990;81:195–199. doi: 10.1111/j.1365-2249.1990.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bancroft G J, Sutton A G, Morris A J, Askonas B A. Production of interferons during experimental African trypanosomiasis. Clin Exp Immunol. 1983;52:135–143. [PMC free article] [PubMed] [Google Scholar]
- 5.Borowy N K, Sternberg J M, Schreiber D, Nonnengasser C, Overath P. Suppressive macrophages occurring in murine Trypanosoma brucei infection inhibit T-cell responses in vivo and in vitro. Parasite Immunol. 1990;12:233–246. doi: 10.1111/j.1365-3024.1990.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 6.Darji A, Sileghem M, Heremans H, Brys L, De Baetselier P. Inhibition of T-cell responsiveness during experimental infections with Trypanosoma brucei: active involvement of endogenous gamma interferon. Infect Immun. 1993;61:3098–3102. doi: 10.1128/iai.61.7.3098-3102.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 8.Flynn J N, Sileghem M. The role of the macrophage in induction of immunosuppression in Trypanosoma congelense infected cattle. Immunology. 1991;74:310–316. [PMC free article] [PubMed] [Google Scholar]
- 9.Ghiotto V, Brun R, Jenni L, Hecker H. Trypanosoma brucei: morphometric changes and loss of infectivity during transformation of bloodstream forms to procyclic culture forms in vitro. Exp Parasitol. 1979;48:447–456. doi: 10.1016/0014-4894(79)90129-2. [DOI] [PubMed] [Google Scholar]
- 10.Hertz C J, Mansfield J M. IFN-γ-dependent nitric oxide production is not linked to resistance in experimental African trypanosomiasis. Cell Immunol. 1999;192:24–32. doi: 10.1006/cimm.1998.1429. [DOI] [PubMed] [Google Scholar]
- 11.Mabbott N, Sternberg J. Bone marrow nitric oxide production and development of anemia in Trypanosoma brucei-infected mice. Infect Immun. 1995;63:1563–1566. doi: 10.1128/iai.63.4.1563-1566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabbott N A, Sutherland I A, Sternberg J M. Trypanosoma brucei is protected from the cytostatic effects of nitric oxide under in vivo conditions. Parasitol Res. 1994;80:687–690. doi: 10.1007/BF00932954. [DOI] [PubMed] [Google Scholar]
- 13.Mabbott N A, Sutherland I A, Sternberg J M. Suppressor macrophages in Trypanosoma brucei infection: nitric oxide is related to both suppressive activity and lifespan in vivo. Parasite Immunol. 1995;17:143–150. doi: 10.1111/j.1365-3024.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 14.McInnes I B, Leung B, Wei X-Q, Gemmell C G, Liew F Y. Septic arthritis following Staphylococcus aureus infection in mice lacking inducible nitric oxide synthase. J Immunol. 1998;160:308–315. [PubMed] [Google Scholar]
- 15.McLintock L M L, Turner C M R, Vickerman K. A comparison of multiplication rates in primary and challenge infections of Trypanosoma brucei bloodstream forms. Parasitology. 1990;101:49–55. doi: 10.1017/s0031182000079749. [DOI] [PubMed] [Google Scholar]
- 16.Olsson T, Bakhiet M, Hojeberg B, Ljungdahl A, Edlund C, Andersson G, Ekre H P, Fung-Leung W P, Mak T, Wigzell H, Fiszer U, Kristensson K. CD8 is critically involved in lymphocyte activation by a T. brucei-released molecule. Cell. 1993;72:715–727. doi: 10.1016/0092-8674(93)90400-k. [DOI] [PubMed] [Google Scholar]
- 17.Samdani A F, Newcamp C, Resink A, Facchinetti F, Hoffman B E, Dawson V L, Dawson T M. Differential susceptibility to neurotoxicity mediated by neurotrophins and neural nitric oxide synthase. J Neurosci. 1997;17:4633–4641. doi: 10.1523/JNEUROSCI.17-12-04633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schleifer K W, Mansfield J M. Suppressor macrophages in African trypanosomiasis inhibit T-cell proliferative responses by nitric oxide and prostaglandins. J Immunol. 1993;151:5492–5503. [PubMed] [Google Scholar]
- 19.Schleifer K W, Filutowicz H, Schopf L R, Mansfield J M. Characterisation of T helper cell responses to the trypanosome variant surface glycoprotein. J Immunol. 1993;150:2910–2919. [PubMed] [Google Scholar]
- 20.Sileghem M, Darji A, Hamers R, Van de Winkel M, De Baetselier P. Dual role of macrophages in the suppression of interleukin 2 production and interleukin 2 receptor expression in trypanosome-infected mice. Eur J Immunol. 1989;19:829–835. doi: 10.1002/eji.1830190508. [DOI] [PubMed] [Google Scholar]
- 21.Sileghem M, Darji A, De Baetselier P. In vitro stimulation of immunosuppression caused by Trypanosoma brucei. Immunology. 1991;73:246–248. [PMC free article] [PubMed] [Google Scholar]
- 22.Sileghem M, Flynn J N. Suppression of interleukin 2 secretion and interleukin 2 receptor expression during tsetse-transmitted trypanosomiasis in cattle. Eur J Immunol. 1992;22:767–773. doi: 10.1002/eji.1830220321. [DOI] [PubMed] [Google Scholar]
- 23.Sternberg J, Mabbott N, Sutherland I, Liew F Y. Inhibition of nitric oxide synthesis leads to reduced parasitemia in murine Trypanosoma brucei infection. Infect Immun. 1994;62:2135–2137. doi: 10.1128/iai.62.5.2135-2137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sternberg J M, McGuigan F. Nitric oxide mediates suppression of T-cell responses in murine Trypanosoma brucei infection. Eur J Immunol. 1992;22:2741–2744. doi: 10.1002/eji.1830221041. [DOI] [PubMed] [Google Scholar]
- 25.Sternberg J M. Immunobiology of African trypanosomiasis. Chem Immunol. 1998;70:186–199. doi: 10.1159/000058706. [DOI] [PubMed] [Google Scholar]
- 26.Sternberg J M, Njogu-Maina N, Gichuki C, Ndung’u J M. Nitric oxide production in vervet monkeys (Cercopithecus aethiops) infected with Trypanosoma brucei. Parasite Immunol. 1998;20:395–397. doi: 10.1046/j.1365-3024.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 27.Taylor K, Lutje V, Mertens B. Nitric oxide synthesis is depressed in Bos indicus cattle infected with Trypanosoma congolense and Trypanosoma vivax and does not mediate T-cell suppression. Infect Immun. 1996;64:4115–4122. doi: 10.1128/iai.64.10.4115-4122.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Turner, C. M. R. Unpublished results.
- 28.Turner, C. M. R. Antigenic variation in Trypanosoma brucei: a holistic view. J. Cell Sci., in press. [DOI] [PubMed]
- 29.Turner C M R, Barry J D. High frequency of antigenic variation in Trypanosoma brucei rhodesiense infections. Parasitology. 1989;99:67–75. doi: 10.1017/s0031182000061035. [DOI] [PubMed] [Google Scholar]
- 30.Turner C M R, Aslam N, Angus S D. Inhibition of growth of Trypanosoma brucei parasites in chronic infections. Parasitol Res. 1996;82:61–66. doi: 10.1007/s004360050069. [DOI] [PubMed] [Google Scholar]
- 31.Vincendeau P, Daulouède S, Veyret B, Darde M L, Bouteille B, Lemesre J P. Nitric oxide-mediated cytostatic activity on Trypanosoma brucei gambiense and Trypanosoma brucei brucei. Exp Parasitol. 1992;75:353–360. doi: 10.1016/0014-4894(92)90220-5. [DOI] [PubMed] [Google Scholar]
- 32.Wei X-Q, Charles I G, Smith A, Ure J, Feng G-J, Huang F-P, Xu D, Muller W, Moncada S, Liew F Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]