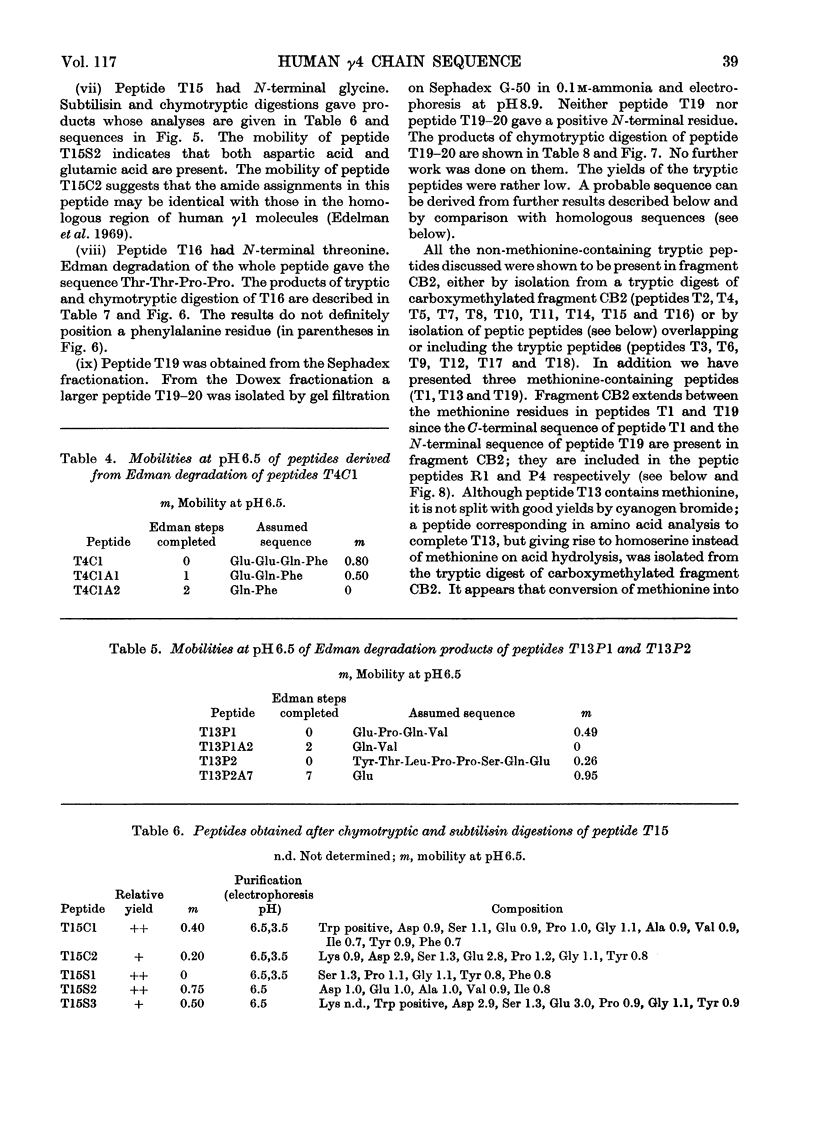
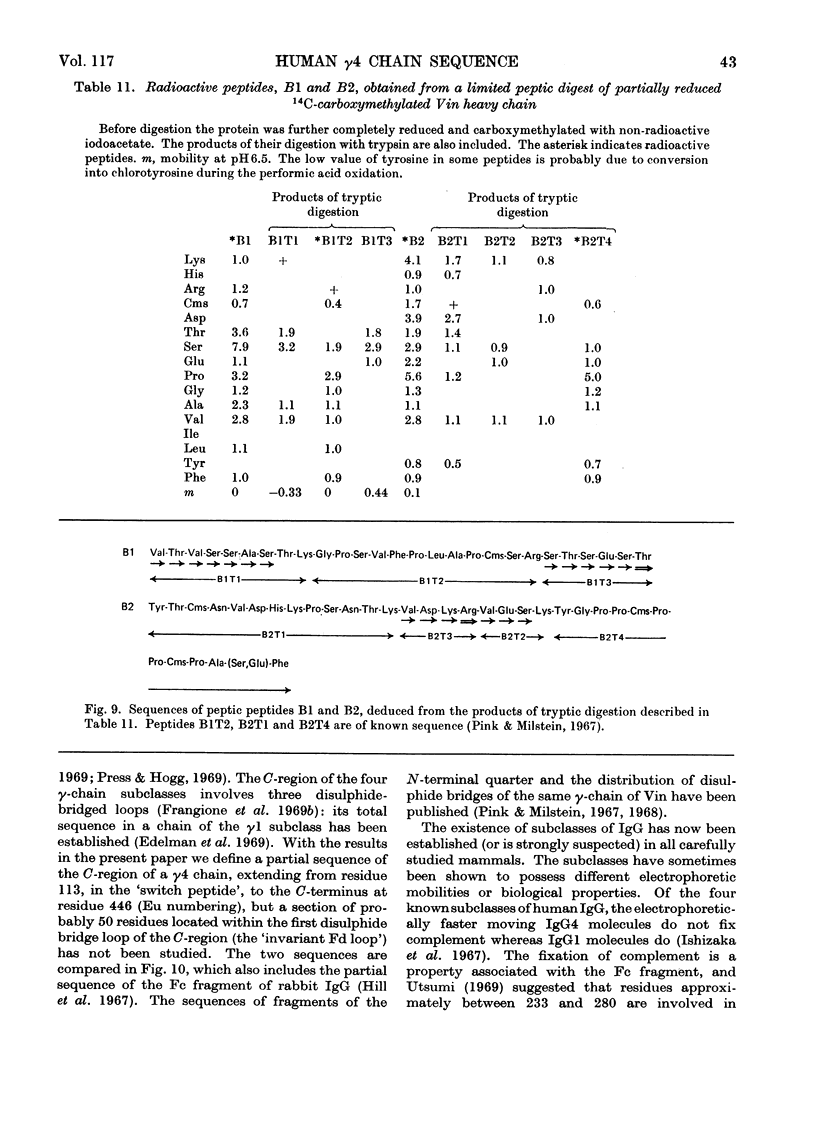
Abstract
The heavy chain of a human myeloma protein (Vin) belonging to the γ4 subclass was subjected to tryptic digestion after reduction and carboxymethylation. Cyanogen bromide fragments were also prepared and all 19 tryptic peptides that account for one of them (the Fc-like fragment) were studied. Selected peptic peptides were isolated and provided evidence for the order of 15 of the tryptic peptides. In addition the sequence of two large peptic peptides derived from two sections of the molecule including all the interchain bridges is presented. Comparison with published data on other chains allows us to propose a sequence of γ4 chains that extends from just before the presumed starting point of the invariable region (at about residue 113) to the C-terminal end of the chain (approx. residue 446), except for a section of about 50 residues. The results of the comparison suggest that the immunoglobulin subclasses have a recent independent evolutionary origin in different species. Implications for complement fixation and for the evolutionary origin of antibody diversity are also discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE PURIFICATION AND AMINO ACID COMPOSITION OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:341–349. doi: 10.1042/bj0890341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Steiner L. A., Porter R. R. The partial sequence of two large peptides from the N-terminal half of heavy chains from normal rabbit immunoglobulin G. Biochem J. 1968 Mar;107(1):79–88. doi: 10.1042/bj1070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Milstein C. Structure and biological properties of immunoglobulins. Adv Immunol. 1967;7:1–89. doi: 10.1016/s0065-2776(08)60126-1. [DOI] [PubMed] [Google Scholar]
- Crowshaw K., Jessup S. J., Ramwell P. W. Thin-layer chromatography of 1-dimethylaminonaphthalene-5-sulphonyl derivatives of amino acids present in superfusates of cat cerebral cortex. Biochem J. 1967 Apr;103(1):79–85. doi: 10.1042/bj1030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUNATSU G. SEPARATION OF TRYPTIC PEPTIDES OF TOBACCO MOSAIC VIRUS AND STRAIN PROTEINS BY AN IMPROVED METHOD OF COLUMN CHROMATOGRAPHY. Biochemistry. 1964 Sep;3:1351–1355. doi: 10.1021/bi00897a027. [DOI] [PubMed] [Google Scholar]
- Frangione B., Franklin E. C., Fudenberg H. H., Koshland M. E. Structural studies of human gamma-G-myeloma proteins of different antigenic subgroups and genetic specificities. J Exp Med. 1966 Oct 1;124(4):715–732. doi: 10.1084/jem.124.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangione B., Milstein C., Franklin E. C. Chemical typing of immunoglobulins. Nature. 1969 Jan 11;221(5176):149–151. doi: 10.1038/221149a0. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C. Partial deletion in the heavy chain disease protein ZUC. Nature. 1969 Nov 8;224(5219):597–599. doi: 10.1038/224597a0. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C., Pink J. R. Structural studies of immunoglobulin G. Nature. 1969 Jan 11;221(5176):145–148. doi: 10.1038/221145a0. [DOI] [PubMed] [Google Scholar]
- GREY H. M., KUNKEL H. G. H CHAIN SUBGROUPS OF MYELOMA PROTEINS AND NORMAL 7S GAMMA-GLOBULIN. J Exp Med. 1964 Aug 1;120:253–266. doi: 10.1084/jem.120.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilse K., Popp R. A. Gene duplication as the basis for amino acid ambiguity in the alpha-chain polypeptides of mouse hemoglobins. Proc Natl Acad Sci U S A. 1968 Nov;61(3):930–936. doi: 10.1073/pnas.61.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L., Ein D. Immunologlobulin lambda chain structure: two genes, one polypeptide chain. Nature. 1968 Nov 23;220(5169):764–767. doi: 10.1038/220764a0. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- Kehoe J. M., Fougereau M. Immunoglobulin peptide with complement fixing activity. Nature. 1969 Dec 20;224(5225):1212–1213. doi: 10.1038/2241212a0. [DOI] [PubMed] [Google Scholar]
- Kelus A. S., Gell P. G. Immunoglobulin allotypes of experimental animals. Prog Allergy. 1967;11:141–184. [PubMed] [Google Scholar]
- Kimura M. Evolutionary rate at the molecular level. Nature. 1968 Feb 17;217(5129):624–626. doi: 10.1038/217624a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J., Edelman G. M. Phylogenetic origins of antibody structure. I. Multichain structure of immunoglobulins in the smooth dogfish (Mustelus canis). J Exp Med. 1965 Sep 1;122(3):601–618. doi: 10.1084/jem.122.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J., Edelman G. M. Polypeptide chains of immunoglobulins from the smooth dogfish (Mustelus canis). Science. 1966 Dec 23;154(3756):1567–1568. doi: 10.1126/science.154.3756.1567. [DOI] [PubMed] [Google Scholar]
- Milstein C. P., Feinstein A. Comparative studies of two types of bovine immunoglobulin G heavy chains. Biochem J. 1968 Apr;107(4):559–564. doi: 10.1042/bj1070559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. Linked groups of residues in immunoglobulin k chains. Nature. 1967 Oct 28;216(5113):330–332. doi: 10.1038/216330a0. [DOI] [PubMed] [Google Scholar]
- Milstein C., Milstein C. P., Feinstein A. Non-allelic nature of the basic sequences of normal immunoglobulin K chains. Nature. 1969 Jan 11;221(5176):151–154. doi: 10.1038/221151a0. [DOI] [PubMed] [Google Scholar]
- Milstein C. The disulphide bridges of immunoglobulin kappa-chains. Biochem J. 1966 Nov;101(2):338–351. doi: 10.1042/bj1010338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Potter M., Lieberman R. Genetics of immunoglobulins in the mouse. Adv Immunol. 1967;7:91–145. doi: 10.1016/s0065-2776(08)60127-3. [DOI] [PubMed] [Google Scholar]
- Prahl J. W. The C-terminal sequences of the heavy chains of human immunoglobulin G myeloma proteins of differing isotopes and allotypes. Biochem J. 1967 Dec;105(3):1019–1028. doi: 10.1042/bj1051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press E. M., Piggot P. J., Porter R. R. The N- and c-terminal amino acid sequences of the heavy chain from a pathological human immunoglobulin IgG. Biochem J. 1966 May;99(2):356–366. doi: 10.1042/bj0990356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O. Halogenation of tyrosine during acid hydrolysis. Biochim Biophys Acta. 1963 May 14;71:468–471. doi: 10.1016/0006-3002(63)91108-9. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Huisman T. H., Shelton J. R., Shelton J. B., Kleihauer E. F., Dozy A. M., Robberson B. Evidence for multiple structural genes for the gamma chain of human fetal hemoglobin. Proc Natl Acad Sci U S A. 1968 Jun;60(2):537–544. doi: 10.1073/pnas.60.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner L. A., Porter R. R. The interchain disulfide bonds of a human pathological immunoglobulin. Biochemistry. 1967 Dec;6(12):3957–3970. doi: 10.1021/bi00864a043. [DOI] [PubMed] [Google Scholar]
- Terry W. D., Fahley J. L., Steinberg A. G. GM and INV factors in subclasses of human IgG. J Exp Med. 1965 Dec 1;122(6):1087–1102. doi: 10.1084/jem.122.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi S. Stepwise cleavage of rabbit immunoglobulin G by papain and isolation of four types of biologically active Fc fragments. Biochem J. 1969 Apr;112(3):343–355. doi: 10.1042/bj1120343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir R. C., Porter R. R., Givol D. Comparison of the C-terminal amino-acid sequence of two horse immunoglobulins IgG and IgG(T). Nature. 1966 Oct 8;212(5058):205–206. doi: 10.1038/212205a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M. Alpha-chains of immunoglobulin A from rabbits of different allotype: composition and N-terminal sequence. Nature. 1969 Aug 9;223(5206):616–617. doi: 10.1038/223616a0. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- van Loghem E., Shuster J., Fudenberg H. H. Gm factors in non-human primates. Vox Sang. 1968;14(2):81–94. doi: 10.1111/j.1423-0410.1968.tb01709.x. [DOI] [PubMed] [Google Scholar]


