Abstract
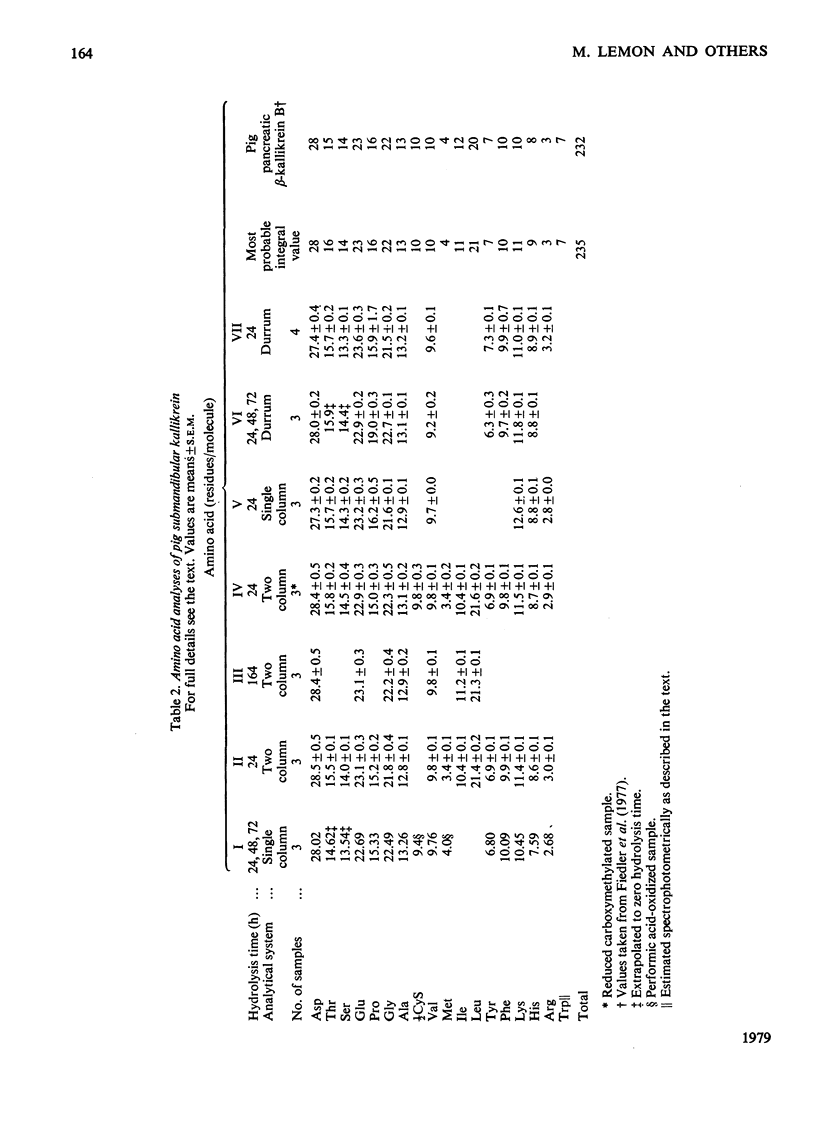
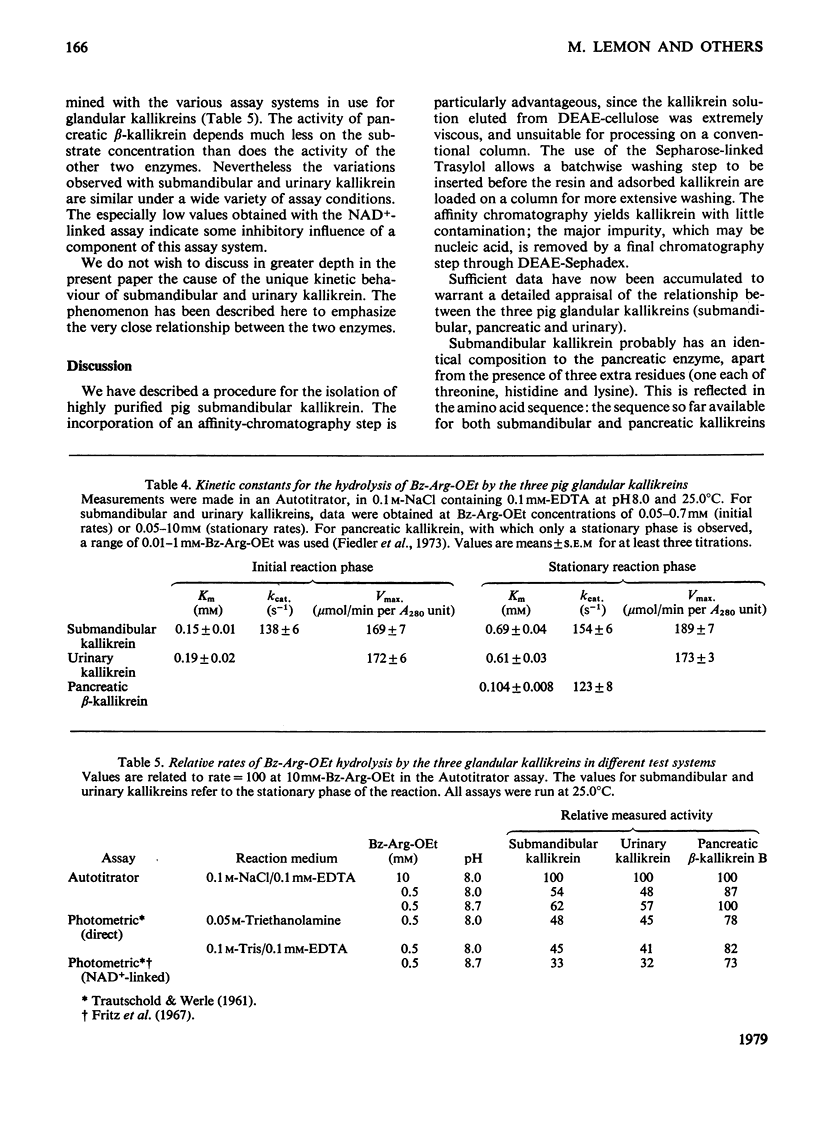
The kallikrein from pig submandibular glands was highly purified, with an overall yield of 31%. Affinity chromatography on bovine basic pancreatic trypsin inhibitor linked to Sepharose 4B was an especially effective step in the purification procedure, giving a purification factor of 80. The enzyme is a single-chain molecule, occurring, as does pig urinary kallikrein, as a major B-form of apparent mol.wt. 39600 and minor amounts of an A-form of apparent mol.wt. 35900; the two forms can be separated by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. The amino acid composition of pig submandibular kallikrein is very similar to, but not quite identical with, that of the two-chain β-kallikrein isolated from pig pancreatic autolysates. Submandibular kallikrein contains notably more glucosamine and hexoses than does pancreatic β-kallikrein. Submandibular kallikrein, and also urinary kallikrein, exhibit an unusual biphasic hydrolysis of substrate esters that is not shared by pancreatic β-kallikrein. For the submandibular enzyme, the Km for the initial reaction phase of the hydrolysis of α-N-benzoyl-l-arginine ethyl ester is 0.15±0.01mm (mean±s.e.m.), but rises to 0.69±0.04mm (mean±s.e.m.) in the stationary reaction phase; the Vmax. does not differ significantly between the two phases. The esterolytic activities of submandibular and urinary kallikreins on a number of esters of different amino acids resemble each other much more closely than those of pancreatic β-kallikrein.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell O. E., Jr, Cain C. E., Sulya L. L., White H. B., Jr The occurrence of polyunsaturated aldehydes in choline-containing phosphoglycerides of a human brain meningioma. Biochim Biophys Acta. 1967 Oct 2;144(2):481–484. doi: 10.1016/0005-2760(67)90183-x. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- FRANCOIS C., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 4. The determination of mannose in hen's-egg albumin by radioisotope dilution. Biochem J. 1962 May;83:335–341. doi: 10.1042/bj0830335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler F., Hirschauer C., Werle E. Anreicherung von Präkallikrein B aus Schweinepankreas und Eigenschaften verschiedener Formen des Pankreaskallikreins. Hoppe Seylers Z Physiol Chem. 1970 Feb;351(2):225–238. [PubMed] [Google Scholar]
- Fiedler F., Hirschauer C., Werle E. Characterization of pig pancreatic kallikreins A and B. Hoppe Seylers Z Physiol Chem. 1975 Dec;356(12):1879–1891. doi: 10.1515/bchm2.1975.356.2.1879. [DOI] [PubMed] [Google Scholar]
- Fiedler F., Leysath G., Werle E. Hdrolysis of amino-acid esters by pig-pancreatic kallikrein. Eur J Biochem. 1973 Jul 2;36(1):152–159. doi: 10.1111/j.1432-1033.1973.tb02895.x. [DOI] [PubMed] [Google Scholar]
- Fiedler F., Müller B., Werle E. Charakterisierung verschiedener Schweinekallikreine mittels Diisopropylfluorophosphat. Hoppe Seylers Z Physiol Chem. 1970 Aug;351(8):1002–1006. [PubMed] [Google Scholar]
- Fiedler F. Pig pancreatic kallikreins A and B. Methods Enzymol. 1976;45:289–303. doi: 10.1016/s0076-6879(76)45027-9. [DOI] [PubMed] [Google Scholar]
- Fiedler F., Werle E. Activation, inhibition, and pH-dependence of the hydrolysis of alpha-N-benzoyl-L-arginine ethyl ester catalyzed by kallikrein from porcine pancreas. Eur J Biochem. 1968 Dec;7(1):27–33. doi: 10.1111/j.1432-1033.1968.tb19569.x. [DOI] [PubMed] [Google Scholar]
- Fritz H., Brey B., Schmal A., Werle E. Verwendung wasserunlöslicher Derivate des Trypsin-Kallikrein-Inhibitors zur Isolierung von Kallikreinen und von Plasmin. Hoppe Seylers Z Physiol Chem. 1969 May;350(5):617–625. [PubMed] [Google Scholar]
- Fritz H., Eckert I., Werle E. Isolierung und Charakterisierung von sialinsäurehaltigem und sialinsäurefreiem Kallikrein aus Schweinepankreas. Hoppe Seylers Z Physiol Chem. 1967 Sep;348(9):1120–1132. [PubMed] [Google Scholar]
- Fritz H., Förg-Brey B. Zur Isolierung von Organ- und Harnkallikreinen durch Affinitätschromatographie. Spezifische Bingung an wasserunlösliche Inhibitorderivate und Dissoziation der Komplexe mit kompetitiven Hemmstoffen (Benzamidin. Hoppe Seylers Z Physiol Chem. 1972 Jun;353(6):901–905. [PubMed] [Google Scholar]
- Fritz H., Hartwich G., Werle E. Uber Proteaseinhibitoren. I. Isolierung und Charakterisierung des Trypsininhibitors aus Pankreasgewebe und Pankreassekret vom Hund. Hoppe Seylers Z Physiol Chem. 1966;345(2):150–167. [PubMed] [Google Scholar]
- HABERMANN E. [Separation and purification of pancreatic kallikrein]. Hoppe Seylers Z Physiol Chem. 1962 Jun 20;328:15–23. doi: 10.1515/bchm2.1962.328.1.15. [DOI] [PubMed] [Google Scholar]
- Kutzbach C., Schmidt-Kastner G. Kallikrein from pig pancreas. Purification, separation of components A and B, and crystallization. Hoppe Seylers Z Physiol Chem. 1972 Jul;353(7):1099–1106. doi: 10.1515/bchm2.1972.353.2.1099. [DOI] [PubMed] [Google Scholar]
- Neurath H., Walsh K. A. Role of proteolytic enzymes in biological regulation (a review). Proc Natl Acad Sci U S A. 1976 Nov;73(11):3825–3832. doi: 10.1073/pnas.73.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUTSCHOLD I., WERLE E. [Spectrophotometric determination of kallikrein and its inactivators]. Hoppe Seylers Z Physiol Chem. 1961 Jun 30;325:48–59. doi: 10.1515/bchm2.1961.325.1.48. [DOI] [PubMed] [Google Scholar]
- WERLE E., TRAUTSCHOLD I. Kallikrein, kallidin, kallikrein inhibitors. Ann N Y Acad Sci. 1963 Feb 4;104:117–129. doi: 10.1111/j.1749-6632.1963.tb17657.x. [DOI] [PubMed] [Google Scholar]
- WHITEHOUSE M. W., ZILLIKEN F. Isolation and determination of neuraminic (sialic) acids. Methods Biochem Anal. 1960;8:199–220. doi: 10.1002/9780470110249.ch5. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wünsch E., Drees F. Zur Synthese des Glucagons. X. Darstellung der Sequenz 22-29. Chem Ber. 1966 Jan;99(1):110–120. doi: 10.1002/cber.19660990119. [DOI] [PubMed] [Google Scholar]
- Zuber M., Sache E. Isolation and characterization of porcine pancreatic kallikrein. Biochemistry. 1974 Jul 16;13(15):3098–3110. doi: 10.1021/bi00712a016. [DOI] [PubMed] [Google Scholar]



