Abstract
Mice deficient in interferon (IFN)-γ or IFN-γ receptor develop progressive and fatal experimental autoimmune encephalomyelitis (EAE). We demonstrate that CD4 T cells lacking IFN-γ production were required to passively transfer EAE, indicating that they were disease-mediating cells in IFN-γ knockout (KO) mice. IFN-γ KO mice accumulated 10–16-fold more activated CD4 T cells (CD4+CD44hi) than wild-type mice in the central nervous system during EAE. CD4+CD44hi T cells in the spleen and central nervous system of IFN-γ KO mice during EAE showed markedly increased in vivo proliferation and significantly decreased ex vivo apoptosis compared with those of wild-type mice. IFN-γ KO CD4+CD44hi T cells proliferated extensively to antigen restimulation in vitro and accumulated larger numbers of live CD4+ CD44hi T cells. IFN-γ completely suppressed proliferation and significantly induced apoptosis of CD4+CD44hi T cells responding to antigen and hence inhibited accumulation of live, activated CD4 T cells. We thus present novel in vivo and in vitro evidence that IFN-γ may limit the extent of EAE by suppressing expansion of activated CD4 T cells.
Keywords: T lymphocytes, apoptosis, autoimmune diseases, animal disease models, knockout mice
Introduction
The role of IFN-γ in experimental autoimmune encephalomyelitis (EAE) was originally thought to be pathogenic 1. However, a large body of evidence has accumulated to indicate a suppressive rather than pathogenic role for IFN-γ in EAE. Thus, administration of anti–IFN-γ Ab 1 or inactivation of either the IFN-γ or IFN-γR gene leads to exacerbation of disease in susceptible strains of mice and conversion of mice from being resistant to being highly susceptible to EAE 2 3 4. Administration of IFN-γ to mice with EAE can inhibit the disease and prevent further relapses 1.
Previous in vitro studies have reported immunosuppressive activities of IFN-γ. For example, we have shown that T cells from IFN-γ knockout (KO) mice display markedly increased in vitro proliferation to Con A and to alloantigens, suggesting a critical role of IFN-γ in suppressing T cell proliferation 5. Anti–IFN-γ Ab enhanced in vitro proliferation of cloned T cells or T cell lines specific to myelin proteins 6 7. IFN-γ KO lymph node and IFN-γR KO spleen cell cultures showed enhanced proliferation to Ag restimulation in vitro 2 8 9. An earlier study reported that IFN-γ is required for anti-CD3–induced cell death of cloned T cells 10. However, none of these studies addressed whether mice lacking an IFN-γ response have increased in vivo T cell proliferation, or decreased T cell apoptosis, or whether this results in accumulation of T cells in vivo.
As EAE is mediated by CD4 T cells 1, we investigated the in vivo proliferation and apoptosis of activated CD4 T cells in IFN-γ KO mice to determine whether IFN-γ has an in vivo role in controlling the expansion of CD4 T cells during EAE. We report here that activated CD4 T cells in the spleen and central nervous system (CNS) of IFN-γ KO mice proliferate more and undergo decreased apoptosis compared with those in wild-type mice. Thus, IFN-γ KO mice accumulate large numbers of activated CD4 T cells in the spleen and CNS during EAE. We further demonstrate that IFN-γ prevents accumulation of activated CD4 T cells in response to in vitro Ag stimulation by both inhibiting proliferation and inducing apoptosis of CD4 T cells.
Materials and Methods
Immunization of Mice.
Homozygous IFN-γ KO C57BL/6 mice were generated by backcrossing 10 generations of the original IFN-γ KO mice onto C57BL/6 background 5. Wild-type C57BL/6 mice were used as controls. 6–10-wk-old mice were injected subcutaneously at the base of the tail with 100 μg of mouse myelin oligodendrocyte glycoprotein peptide 35–55 (MOG35–55; Genosys Biotechnologies) in CFA containing 5 mg/ml of killed Mycobacterium tuberculosis (H37Ra) and 0.5 mg/ml M. butyricum (Difco Labs.). Mice were also injected intraperitoneally with 200 ng of pertussis toxin (List Biological Labs.). Mice were given an identical booster on day 7 of initial immunization.
Adoptive Transfer of EAE.
Spleen cells from MOG35–55-immunized mice were cultured with 10 μg/ml MOG35–55 for 48 h in T cell medium (RPMI 1640 with 7.5% FCS, 0.01 M Hepes, 5 × 10−5 M 2-ME, 0.292 mg/ml glutamine, and antibiotics) and 80 U/ml of recombinant murine IL-2 (R & D Systems). CD4 T cells were depleted from cultured spleen cells using a MACS™ magnetic separation column (Miltenyi Biotec). Routinely, 99% depletion of CD4 T cells was achieved. 50 million whole spleen cells or CD4 T cell–depleted spleen cells were injected intravenously into each mouse.
Isolation of Mononuclear Cells from CNS.
Mononuclear cells in the CNS were isolated using gradient centrifugation as described 11. In brief, mice were perfused with 20 ml of PBS via the retroorbital veins to eliminate peripheral blood. The dissociated CNS tissue was centrifuged in a Percoll gradient. Mononuclear cells at the interface of 70–37% Percoll were collected and stained for phenotype analysis.
In Vivo 5-Bromo-2′-Deoxyuridine Uptake.
Mice were injected intraperitoneally with 0.8 mg of 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich) four times within 48 h. Spleen cells and CNS mononuclear cells were stained with anti-CD4–Cy-Chrome and anti-CD44–PE (PharMingen). Stained cells were fixed in 70% ethanol, resuspended in 1% paraformaldehyde containing 0.01% Tween-20. The cells were treated with 50 Kunitz units/ml DNase and incubated with 0.25 μg of anti-BrdU–FITC (B44; Becton Dickinson). BrdU incorporation was analyzed on gated CD4+CD44hi T cells.
Apoptosis Assay.
For ex vivo detection of apoptosis, freshly isolated spleen cells and CNS mononuclear cells were stained as described below. For in vitro induction of apoptosis, spleen cells from MOG35–55-immunized mice were cultured at 5 × 106/ml (0.2 ml/well) in 96-well plates with MOG35–55 in T cell medium and IL-2 (80 U/ml). IFN-γ (50 ng/ml; R & D Systems) or anti–IFN-γ mAb (clone XMG1.2, rat IgG1; American Type Culture Collection; 10 μg/ml) was added at the beginning of cultures. A rat IgG1 (antiperoxidase; from C. Dean, Institute of Cancer Research, Surrey, UK) was used as an isotype control for anti–IFN-γ mAb. At various time points, cells were stained with anti-CD4–allophycocyanin and anti-CD44–PE and resuspended in annexin binding buffer. The cells were stained for 15 min with 0.05 μg of FITC-labeled annexin V (R & D Systems) at room temperature. Immediately before collection, 0.25 μg of propidium iodide (PI) was added. Apoptosis was analyzed on gated CD4+CD44hi T cells.
Cell Labeling with Carboxyfluorescein Succinimidyl Ester and In Vitro Proliferation of CD4 T Cells.
Splenic CD4 T cells were purified using magnetic beads conjugated with anti-CD4 Ab and a magnetic column (Miltenyi Biotec). Purified CD4 T cells (90–95% purity) were labeled with carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) as described 12. Labeling efficiency exceeded 99%. CFSE-labeled, purified CD4 T cells were cultured in T cell medium (105/well) with either CD4 T cell–depleted spleen cells (4 × 105/well) or activated B cells (4 × 105/well) as APCs. Cells were cultured in T cell medium alone or containing MOG35–55 or human osteocalcin peptide (OC7–19). Cells were harvested at time points indicated (see Fig. 4) and stained with anti-CD4–allophycocyanin and anti-CD44–PE. CFSE fluorescence profile was analyzed on gated CD4+CD44hi T cells.
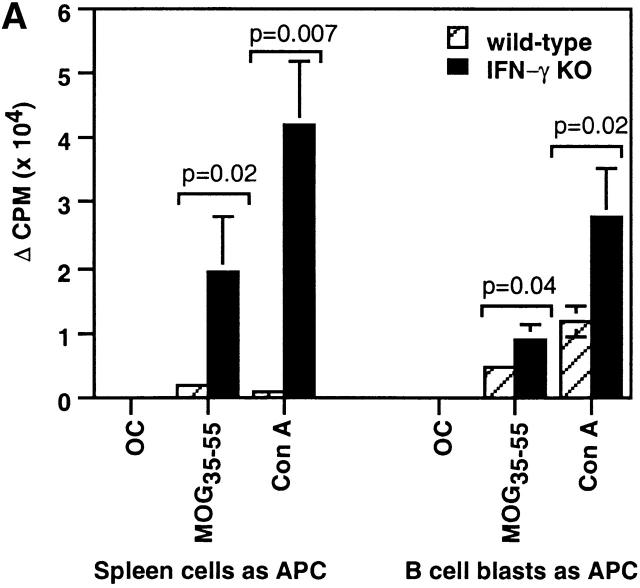
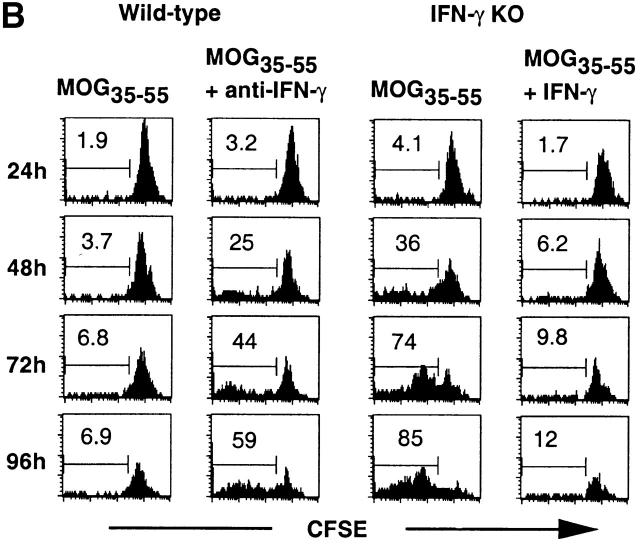
Figure 4.
Increased in vitro proliferation of activated CD4 T cells of IFN-γ KO mice to MOG35–55. (A) CD4 T cells were purified from spleens of mice immunized with MOG35–55 for 2 wk and cultured in T cell medium alone or in the presence of OC7–19, MOG35–55, or Con A with their own CD4 T cell–depleted spleen cells or mitomycin C–treated activated B cells as APCs. The cells were cultured for 65 h and pulsed with 0.2 μCi [3H]thymidine for the last 16 h (two experiments; n = 4). (B) Kinetic analysis of effects of IFN-γ on CD4+CD44hi T cell proliferation to MOG35–55 in vitro. CFSE-labeled, purified CD4 T cells from MOG35–55-immunized mice were cultured with MOG35–55 and CD4 T cell–depleted spleen cells as APCs in the presence of anti–IFN-γ or IFN-γ. Representative histograms from three experiments (n = 4) show proliferation of CD4+CD44hi T cells.
Results
CD4 T Cells Were Required for Adoptive Transfer of EAE in IFN-γ KO Mice.
Consistent with previous studies on the course of EAE in mice lacking IFN-γ or IFN-γR 2 3 4, we found that IFN-γ KO C57BL/6 mice were highly susceptible to MOG and myelin basic protein–induced EAE and had a chronic, progressive, and more severe course of disease than wild-type mice (data not shown).
Most CD4 T cell lines that passively transfer EAE make IFN-γ 1. To determine whether CD4 T cells that are deficient in IFN-γ production function as effector cells in EAE, we tested whether CD4 T cell–depleted spleen cells can passively transfer EAE. In both wild-type and IFN-γ KO recipients, mice that received whole spleen cells developed EAE (Fig. 1), while depletion of CD4 T cells from spleen cells completely abolished the transfer of EAE in both wild-type and IFN-γ KO mice (Fig. 1). These results clearly indicate that in IFN-γ KO mice, CD4 T cells are required for passive transfer of EAE and suggest that they are effector cells in actively induced EAE.
Figure 1.
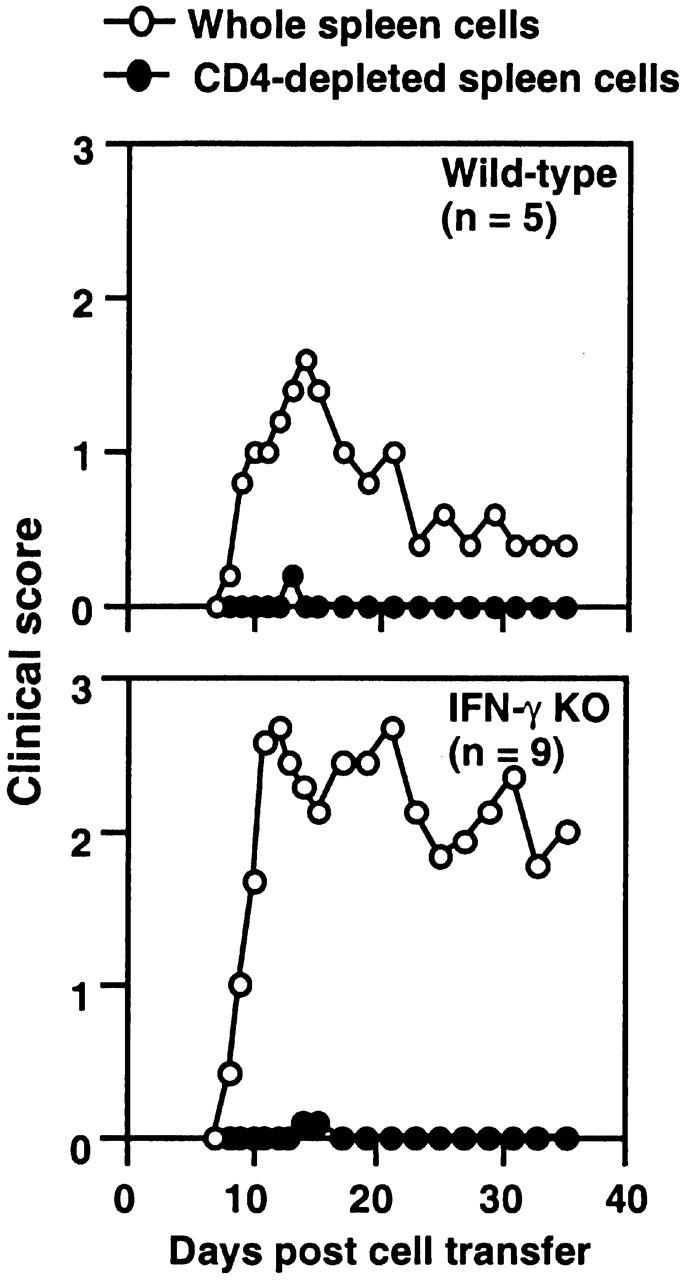
CD4 T cells from IFN-γ KO mice were required for adoptive transfer of EAE. Whole spleen cells from MOG35–55-immunized mice were restimulated with 10 μg/ml MOG35–55 for 48 h. 50 million whole spleen cells or CD4 T cell–depleted spleen cells were transferred into naive mice (wild-type cells to wild-type and IFN-γ KO cells to IFN-γ KO recipients). Development of EAE was monitored for 5 wk (pooled results of two experiments).
Increased In Vivo Accumulation of Activated CD4 T Cells in IFN-γ KO Mice with EAE.
The kinetics of accumulation of activated CD4 T cells was followed during the course of EAE. Ag-experienced CD4 T cells express high levels of CD44 after activation 13. We used CD44hi expression as a marker for activated CD4 T cells. CD4 T cells that were CD44hi were also CD45RBlow and CD62Llow. This is consistent with an activated phenotype 14. 2 wk after immunization, the population of CD4+CD44hi T cells in the spleen markedly increased from an average of 20% of CD4 T cells to 38% in wild-type and to 57% in IFN-γ KO mice. The extent of the increase in absolute number of CD4+CD44hi T cells was greater in IFN-γ KO mice. Approximately threefold more CD4+CD44hi T cells were found in the spleens of IFN-γ KO than wild-type mice (Fig. 2 A). At all time points after immunization, the number of splenic CD4+CD44hi T cells in IFN-γ KO mice was significantly greater than that in wild-type mice (Fig. 2 A).
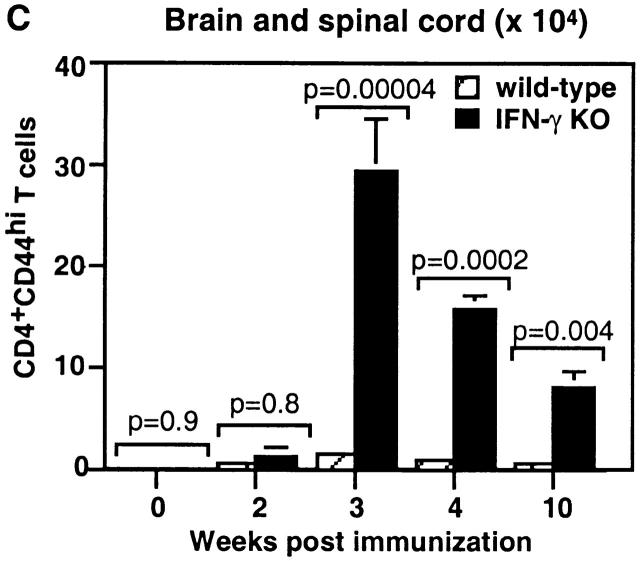
Figure 2.
IFN-γ KO mice accumulated a larger number of activated CD4 T cells in the spleen and CNS during EAE. The absolute number of live CD4+CD44hi T cells was determined based on total numbers of live cells and percentage of CD4+CD44hi T cells analyzed by flow cytometry. (A) Kinetics of accumulation of CD4+CD44hi T cells in the spleens of IFN-γ KO mice. (B) The CD4 T cells in the CNS in IFN-γ KO mice with EAE were more skewed toward the activated phenotype (CD44hi) compared with those in wild-type mice. CD4 T cells from mice on day 21 of immunization were analyzed. The marker of CD44hi on CD4 T cells in mice with EAE was set up based on expression of CD44 on splenic CD4 T cells in an unimmunized wild-type mouse. (C) Kinetics of accumulation of CD4+CD44hi T cells in the CNS of IFN-γ KO mice during EAE. Pooled results of three experiments (n = 6–10 at each time point).
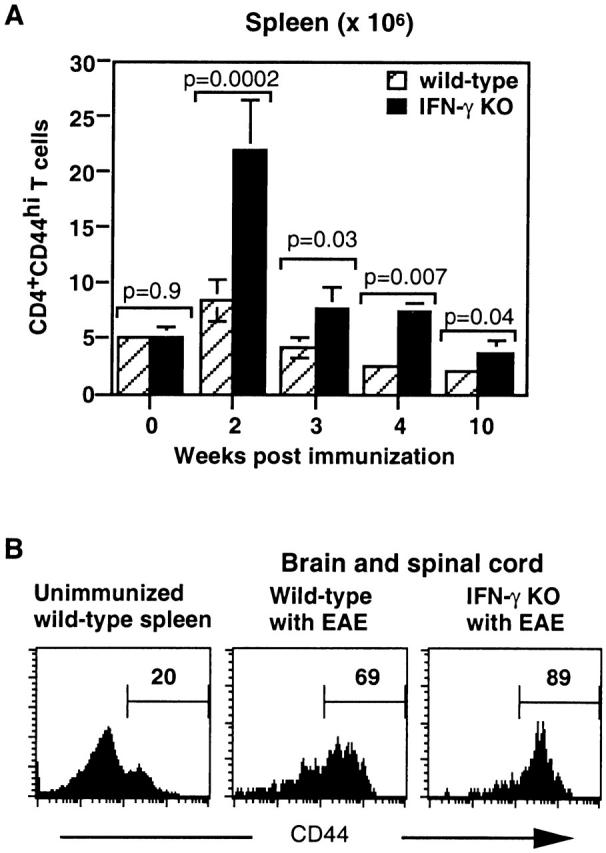
More strikingly, the difference in the number of activated CD4 T cells between IFN-γ KO and wild-type mice was much greater in the CNS than in the spleen. In the CNS of IFN-γ KO mice, 70–95% of CD4 T cells were CD44hi, compared with 50–70% of CD4 T cells in wild-type mice (Fig. 2 B). 3 wk after immunization, the absolute number of CD4+CD44hi T cells in the CNS of IFN-γ KO mice was 16-fold greater than in wild-type mice (Fig. 2 C). The number of CD4+CD44hi T cells in the CNS of mice with EAE decreased with time of disease in both wild-type and IFN-γ KO mice but diminished with delayed kinetics in IFN-γ KO mice. At 4 wk of immunization, there were 13-fold more CD4+CD44hi T cells in the CNS of IFN-γ KO mice, and at 10 wk, there were 10-fold more than in wild-type mice (Fig. 2 C).
Increased In Vivo Proliferation of CD4+CD44hi T Cells in IFN-γ KO Mice during EAE.
IFN-γ has been shown to inhibit proliferation of T cells in vitro 5 6 7. A unique tool, the IFN-γ KO mice have allowed us to extend these in vitro observations in vivo to explore the effect of IFN-γ on CD4 T cells in a CD4 T cell–mediated disease. We investigated the in vivo proliferation of CD4+CD44hi T cells during EAE using a BrdU assay. Compared with wild-type mice, the spleen and CNS of IFN-γ KO mice had a significantly higher percentage of CD4+CD44hi T cells incorporating BrdU (Fig. 3 A), indicating that they were proliferating in vivo. This gave rise to a greater number of proliferating CD4+CD44hi T cells in the spleen and CNS in IFN-γ KO mice during EAE. Between 2 and 4 wk after immunization, the number of CD4+CD44hi T cells incorporating BrdU in IFN-γ KO mice was 2.5–5-fold in the spleen and 4.8–6-fold in the CNS of that in wild-type mice.
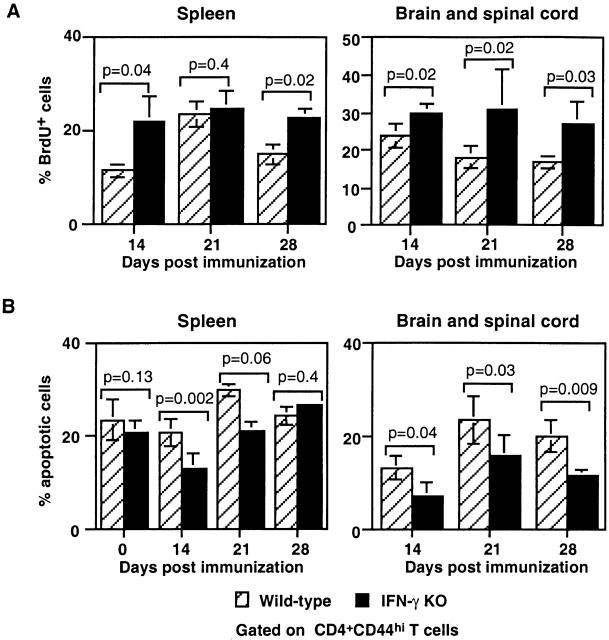
Figure 3.
Enhanced in vivo proliferation and decreased ex vivo apoptosis of activated CD4 T cells in IFN-γ KO mice with EAE. (A) In vivo proliferation of CD4+CD44hi T cells was measured using BrdU incorporation assay. A greater percentage of CD4+CD44hi T cells in both the spleen and CNS of IFN-γ KO mice incorporated BrdU (Pooled data of three experiments; n = 6–8 at each time point). (B) Decreased ex vivo apoptosis of CD4+CD44hi T cells in IFN-γ KO mice with EAE. Freshly isolated spleen cells and CNS mononuclear cells were stained with annexin V–FITC and PI. The figure shows that a smaller percentage of CD4+CD44hi T cells in the spleens of IFN-γ KO mice underwent apoptosis during the first 3 wk after immunization. In the CNS, at all the time points assayed, the percentage of apoptotic CD4+CD44hi T cells in IFN-γ KO mice was smaller than that in wild-type mice (pooled data of three experiments; n = 6–10 at each time point).
Decreased Apoptosis of Activated CD4 T Cells in the CNS of IFN-γ KO Mice with EAE.
Another possible mechanism for controlling expansion of activated CD4 T cells in the CNS is apoptosis of this population. We have found that IFN-γ KO mice failed to induce apoptosis of activated CD4 T cells during infection with M. bovis Bacillus Calmette-Guérin (BCG) 15. This prompted us to consider this possibility in this EAE model. Freshly isolated spleen cells and mononuclear cells from the CNS of mice with EAE were analyzed for apoptosis using an annexin/PI flow cytometric assay. In the spleen at 14 and 21 d after immunization, IFN-γ KO mice had a significantly lower percentage of CD4+CD44hi T cells that were apoptotic compared with those in wild-type mice (Fig. 3 B). Additionally, IFN-γ KO mice had a significantly lower percentage of CD4+ CD44hi T cells that were apoptotic in the CNS. On day 28 after immunization, there were 20 ± 3.4% apoptotic CD4+CD44hi T cells in the CNS of wild-type mice compared with 11.5 ± 1% apoptotic in the CNS of IFN-γ KO mice (Fig. 3 B).
Thus, in vivo activated CD4 T cells of IFN-γ KO mice responding to MOG35–55 immunization displayed increased in vivo proliferation and decreased apoptosis. This likely contributes to the increased number of activated CD4 T cells seen in IFN-γ KO mice during EAE.
Enhanced Response of IFN-γ KO CD4 T Cells to MOG35–55 In Vitro and Inhibition by IFN-γ.
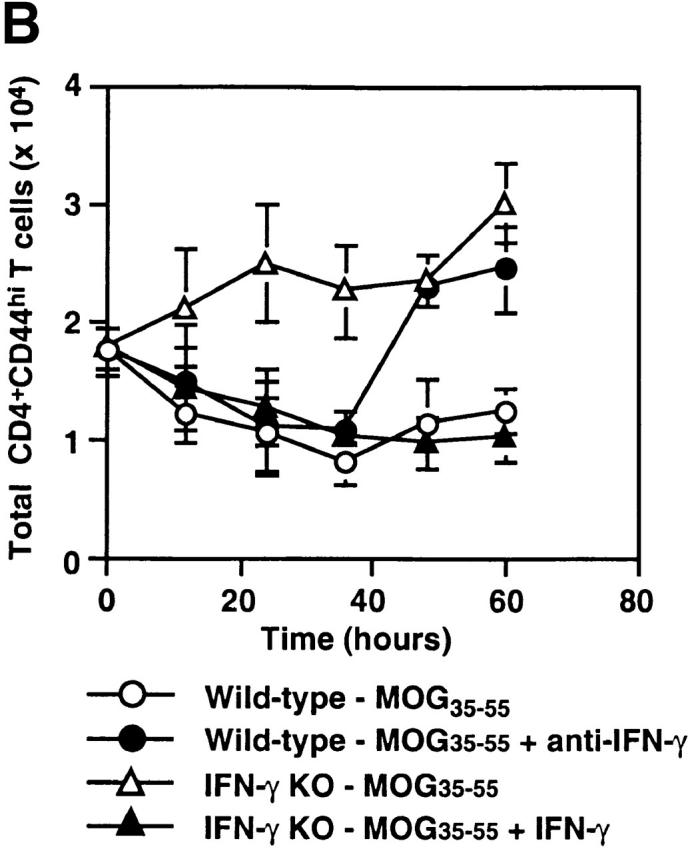
Consistent with previous findings 2 9, we observed markedly enhanced proliferation of IFN-γ KO spleen cells to MOG35–55 restimulation in vitro and unresponsiveness of wild-type cells (Fig. 4 A). We considered that APCs in the spleens of wild-type mice might be suppressing CD4 T cell proliferation and used MOG35–55-pulsed, LPS-activated B cells as APCs to stimulate wild-type CD4 T cells. In contrast to CD4 T cells cultured with their own spleen cells as APCs, CD4 T cells purified from immunized wild-type mice significantly proliferated to MOG35–55 when cultured with activated B cells (Fig. 4 A). Thus, it appears that wild-type APCs are suppressing CD4 T cell proliferation.
We proceeded to study the effects of IFN-γ on proliferation of activated CD4 T cells in vitro in response to the priming Ag, MOG35–55. We used a CFSE labeling assay to specifically measure the kinetics of activated CD4 T cell proliferation in whole spleen cell culture. Neutralization of IFN-γ significantly increased the percentage of dividing wild-type CD4+CD44hi T cells to MOG35–55, especially at later time points (Fig. 4 B). Conversely, addition of IFN-γ to IFN-γ KO cultures inhibited proliferation of CD4+ CD44hi T cells. Thus, IFN-γ suppresses proliferation of activated CD4 T cells during in vitro Ag restimulation.
Induction of Apoptosis of CD4+CD44hi T Cells by IFN-γ In Vitro.
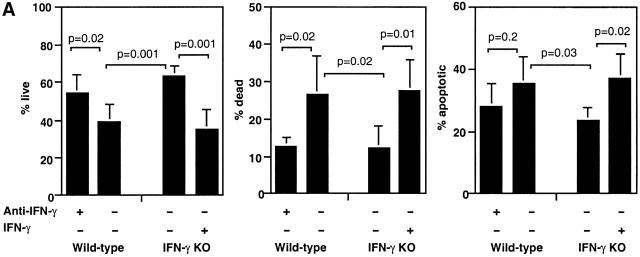
We then tested the effect of IFN-γ on apoptosis and death of CD4+CD44hi T cells in response to Ag restimulation. IFN-γ KO cultures had 63 ± 5% live CD4+CD44hi T cells at 60 h of culture compared with 39 ± 9% in wild-type cultures (Fig. 5 A). Addition of IFN-γ to IFN-γ KO cultures significantly decreased the number of live CD4+CD44hi T cells to 35 ± 10%. At the same time, the percentage of apoptotic and dead CD4+CD44hi T cells increased significantly (Fig. 5 A). Conversely, neutralization of IFN-γ in wild-type cultures significantly increased the number of live CD4+CD44hi T cells to 54 ± 9% and decreased the percentage of apoptotic and significantly decreased the percentage of dead CD4+CD44hi T cells (Fig. 5 A). A second apoptosis assay, the TUNEL (TdT-mediated dUTP-biotin nick-end labeling) method, was also used to measure in vitro apoptosis of CD4+CD44hi T cells and produced similar results (not shown). These data indicate that IFN-γ induces apoptosis and death of activated CD4 T cells during in vitro restimulation with Ag.
Figure 5.
IFN-γ suppressed accumulation of live CD4+CD44hi T cells in response to MOG35–55. (A) Whole spleen cells from MOG35–55-immunized mice were cultured with MOG35–55 for 60 h in T cell medium and IL-2 in the presence of anti–IFN-γ or IFN-γ. The figure shows percentage of live, dead, and apoptotic CD4+CD44hi T cells as determined by annexin/PI assay (pooled data of three experiments; n = 8 in wild-type and n = 6 in IFN-γ KO group). (B) Whole spleen cells were cultured and analyzed as described above. Live CD4+CD44hi T cells were determined based on the number of total cells and percentage of live CD4+CD44hi T cells (pooled data of two experiments; n = 4).
The Suppressive Effect of IFN-γ on In Vitro Accumulation of Activated CD4 T Cells Responding to Ag.
We counted the total number of cells during in vitro restimulation with MOG35–55 and calculated the absolute number of live CD4+CD44hi T cells. At the end of a 60-h culture, threefold more live CD4+CD44hi T cells were recovered from IFN-γ KO than from wild-type cultures (Fig. 5 B). Addition of IFN-γ to IFN-γ KO cells led to a significant decrease in the number of live CD4+CD44hi T cells. In contrast, the number of live CD4+CD44hi T cells from wild-type mice decreased with time of culture, but in the presence of anti-IFN-γ, the number of live CD4+CD44hi T cells increased significantly compared with the starting number (Fig. 5 B).
Discussion
EAE is mediated by autoreactive CD4 T cells 1. It has been suggested that apoptotic deletion of autoreactive T cells is related to downregulation of Ag-reactive T cells during spontaneous recovery from EAE 16 17. A striking feature of EAE in IFN-γ KO or IFN-γR KO mice is that the disease is progressive and fatal. Our study focused on the kinetics of accumulation of activated CD4 T cells in the CNS of mice deficient in IFN-γ production. In IFN-γ KO mice, the activated CD4 T cells persisted in the CNS in large numbers until the termination of experiments 10 wk after Ag challenge. Our results suggest that IFN-γ KO mice have a defect in eliminating disease-mediating CD4 T cells. The persistence of large numbers of activated CD4 T cells in the CNS is the likely cause for the chronic progressive disease course in IFN-γ KO mice.
Although several studies have shown that IFN-γ inhibits in vitro proliferation of myelin protein–specific cloned T cells or T cell lines 6 7 and in vitro proliferation of primary lymphocytes from mice with EAE in a bulk cell culture 2 8 9, there have been no reports addressing the role of IFN-γ on in vivo proliferation of CD4 T cells in mice with EAE. Our data provide critical evidence that there is increased in vivo proliferation of activated CD4 T cells in IFN-γ KO mice with EAE. However, increased proliferation of T cells does not necessarily lead to increased accumulation of T cells, as another mechanism for controlling T cell expansion is increased death. We demonstrate here for the first time that in IFN-γ KO mice with EAE-activated CD4 T cells taken ex vivo from the CNS and spleen are undergoing decreased apoptosis. We also demonstrate for the first time that addition of IFN-γ to activated CD4 T cells from IFN-γ KO mice induced apoptosis of these cells in response to Ag restimulation. Thus, the mechanism by which IFN-γ inhibits EAE is both by inhibiting proliferation and by inducing apoptosis of activated CD4 T cells. The absence of IFN-γ results in a massive accumulation of activated CD4 T cells in the CNS and spleen after challenge with myelin proteins.
We and others have observed increased inflammation and increased demyelination in the CNS of IFN-γ KO mice 9, suggesting an IFN-γ–independent pathway that can mediate destruction of myelin. Two recent reports have demonstrated that there are large numbers of neutrophils in the CNS of mice lacking IFN-γ or IFN-γR 2 9. IFN-γ KO mice express chemokines in the CNS that attract neutrophils 9. Another report has shown that depletion of mice with antigranulocyte Ab inhibits EAE in both SJL and IFN-γR KO mice 18. The relationship between large numbers of CD4 T cells and neutrophils in the CNS and the role of each type of cell in mediating myelin destruction is not clear. It is possible that the activated CD4 T cells may produce chemokines that attract neutrophils or vice versa.
We show in another study, by Dalton et al., published in this issue that IFN-γ is not acting directly on CD4 T cells but rather is acting indirectly through activated macrophages and nitric oxide (NO) 15. Whether activated macrophages in the spleen or CNS play a role in induction of apoptosis by IFN-γ during EAE is currently under investigation. Our preliminary evidence in the EAE model is that NO may play a role in suppressing proliferation of activated CD4 T cells. There is in vitro evidence that NO plays a role in inhibiting Ag-specific T cell proliferation 7 8 and in inducing apoptosis of encephalitogenic T cell clones 19. Furthermore, EAE is exacerbated in inducible NO synthase KO mice 20 21. We hypothesize that during EAE, CD4 T cells making IFN-γ infiltrate the CNS and activate macrophages, microglia, or dendritic cells to make NO. NO may then induce apoptosis of activated CD4 T cells, shutting down the immune response and inducing a remission of disease. In the absence of IFN-γ, the mechanism to eliminate activated CD4 T cells is impaired, the activated CD4 T cells and neutrophils accumulate, and the disease is chronic and progressive.
Acknowledgments
This work was supported by a Trudeau Institute grant (IHP-85 to D.K. Dalton).
References
- Begolka W.S., Miller S.D. Cytokines as intrinsic and exogenous regulators of pathogenesis in experimental autoimmune encephalomyelitis. Res. Immunol. 1998;149:771–781. doi: 10.1016/s0923-2494(99)80004-2. [DOI] [PubMed] [Google Scholar]
- Willenborg D.O., Fordham S., Bernard C.C., Cowden W.B., Ramshaw I.A. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- Ferber I.A., Brocke S., Taylor-Edwards C., Ridgway W., Dinisco C., Steinman L., Dalton D., Fathman C.G. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J. Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Krakowski M., Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- Dalton D.K., Pitts-Meek S., Keshav S., Figari I.S., Bradley A., Stewart T.A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Duong T.T., Finkelman F.D., Strejan G.H. Effect of interferon-gamma on myelin basic protein-specific T cell line proliferation in response to antigen-pulsed accessory cells. Cell. Immunol. 1992;145:311–323. doi: 10.1016/0008-8749(92)90334-l. [DOI] [PubMed] [Google Scholar]
- van der Veen R.C., Dietlin T.A., Dixon Gray J., Gilmore W. Macrophage-derived nitric oxide inhibits the proliferation of activated T helper cells and is induced during antigenic stimulation of resting T cells. Cell. Immunol. 2000;199:43–49. doi: 10.1006/cimm.1999.1597. [DOI] [PubMed] [Google Scholar]
- Willenborg D.O., Fordham S.A., Staykova M.A., Ramshaw I.A., Cowden W.B. IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissuea possible role for nitric oxide. J. Immunol. 1999;163:5278–5286. [PubMed] [Google Scholar]
- Tran E.H., Prince E.N., Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J. Immunol. 2000;164:2759–2768. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- Liu Y., Janeway C., Jr. Interferon gamma plays a critical role in induced cell death of effector T cella possible third mechanism of self-tolerance. J. Exp. Med. 1990;172:1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowski M.L., Owens T. The central nervous system environment controls effector CD4+ T cell cytokine profile in experimental allergic encephalomyelitis. Eur. J. Immunol. 1997;27:2840–2847. doi: 10.1002/eji.1830271115. [DOI] [PubMed] [Google Scholar]
- Lyons A.B., Parish C.R. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Budd R.C., Cerottini J.C., Horvath C., Bron C., Pedrazzini T., Howe R.C., MacDonald H.R. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J. Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- Bell E.B., Sparshott S.M., Bunce C. CD4+ T-cell memory, CD45R subsets and the persistence of antigen—a unifying concept. Immunol. Today. 1998;19:60–64. doi: 10.1016/s0167-5699(97)01211-5. [DOI] [PubMed] [Google Scholar]
- Dalton D.K., Haynes L., Chu C.-Q., Swain S.L., Wittmer S. Interferon γ eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J. Exp. Med. 2000;191:117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabi Z., McCombe P.A., Pender M.P. Antigen-specific down-regulation of myelin basic protein-reactive T cells during spontaneous recovery from experimental autoimmune encephalomyelitisfurther evidence of apoptotic deletion of autoreactive T cells in the central nervous system. Int. Immunol. 1995;7:967–973. doi: 10.1093/intimm/7.6.967. [DOI] [PubMed] [Google Scholar]
- Xiao B.G., Huang Y.M., Xu L.Y., Ishikawa M., Link H. Mechanisms of recovery from experimental allergic encephalomyelitis induced with myelin basic protein peptide 68-86 in Lewis ratsa role for dendritic cells in inducing apoptosis of CD4+ T cells. J. Neuroimmunol. 1999;97:25–36. doi: 10.1016/s0165-5728(99)00041-7. [DOI] [PubMed] [Google Scholar]
- McColl S.R., Staykova M.A., Wozniak A., Fordham S., Bruce J., Willenborg D.O. Treatment with anti-granulocyte antibodies inhibits the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 1998;161:6421–6426. [PubMed] [Google Scholar]
- Zettl U.K., Mix E., Zielasek J., Stangel M., Hartung H.P., Gold R. Apoptosis of myelin-reactive T cells induced by reactive oxygen and nitrogen intermediates in vitro. Cell. Immunol. 1997;178:1–8. doi: 10.1006/cimm.1997.1113. [DOI] [PubMed] [Google Scholar]
- Fenyk-Melody J.E., Garrison A.E., Brunnert S.R., Weidner J.R., Shen F., Shelton B.A., Mudgett J.S. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J. Immunol. 1998;160:2940–2946. [PubMed] [Google Scholar]
- Sahrbacher U.C., Lechner F., Eugster H.P., Frei K., Lassmann H., Fontana A. Mice with an inactivation of the inducible nitric oxide synthase gene are susceptible to experimental autoimmune encephalomyelitis. Eur. J. Immunol. 1998;28:1332–1338. doi: 10.1002/(SICI)1521-4141(199804)28:04<1332::AID-IMMU1332>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]