Abstract
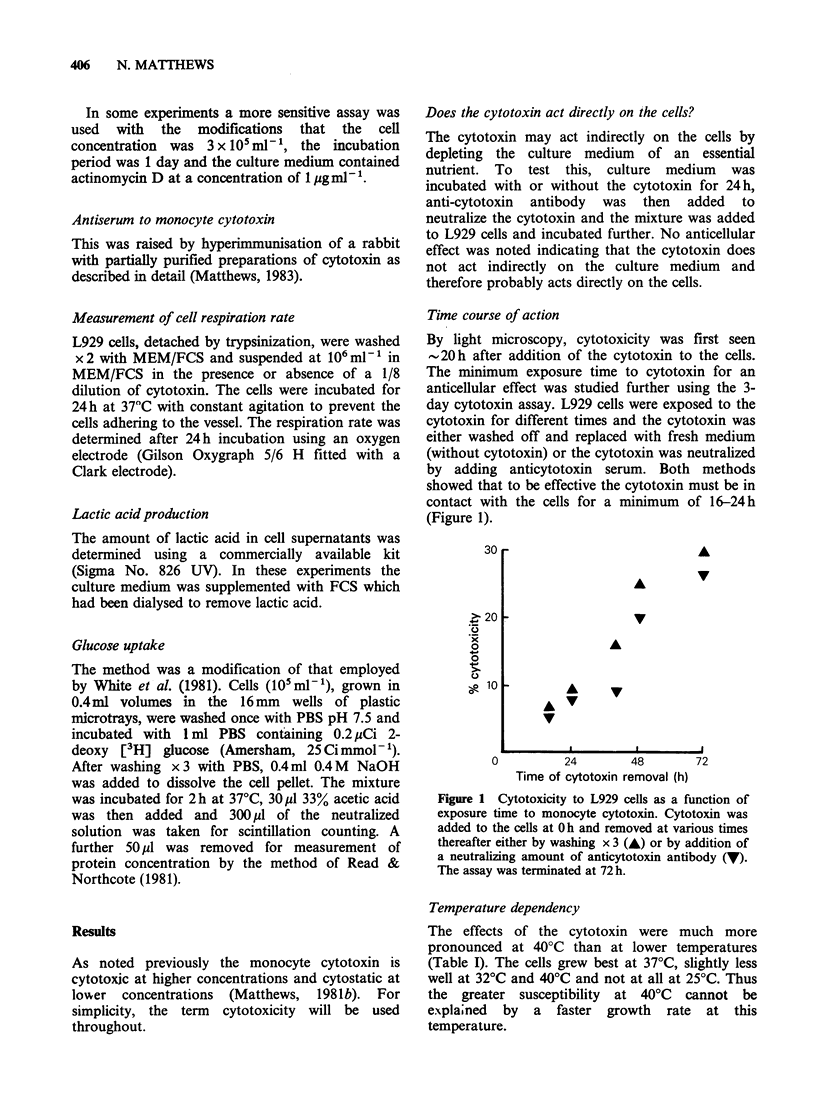
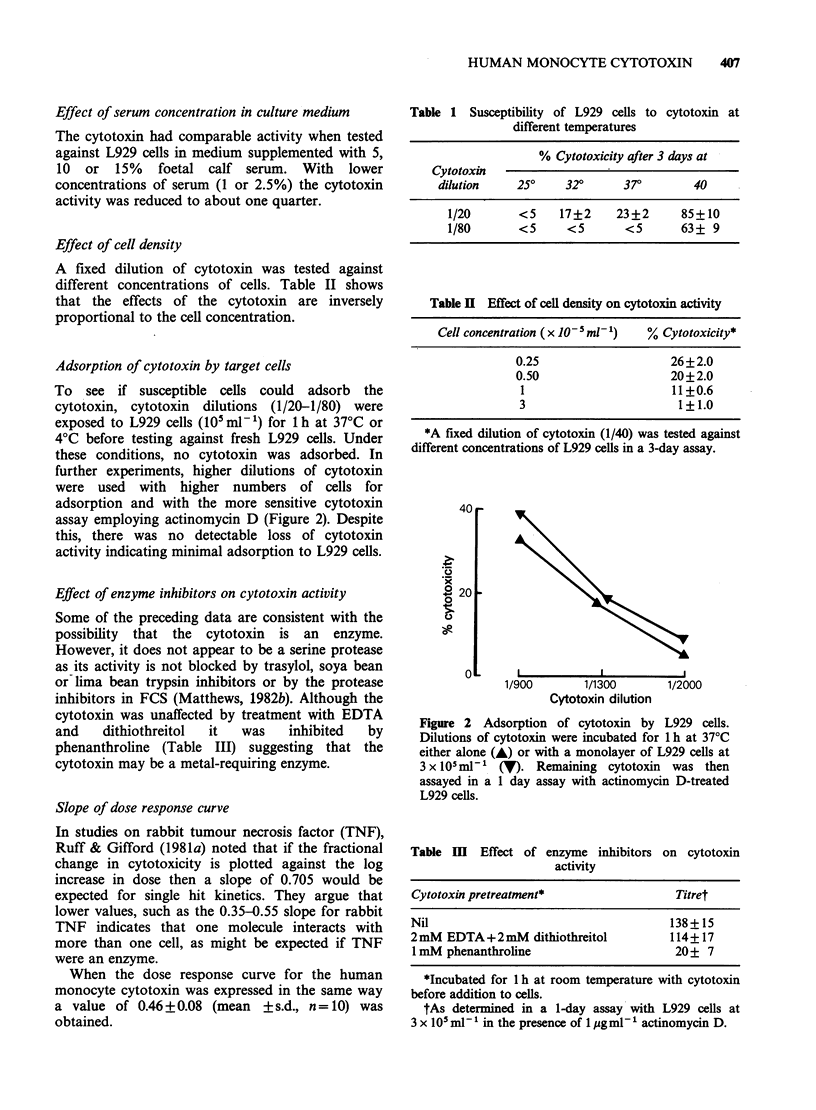
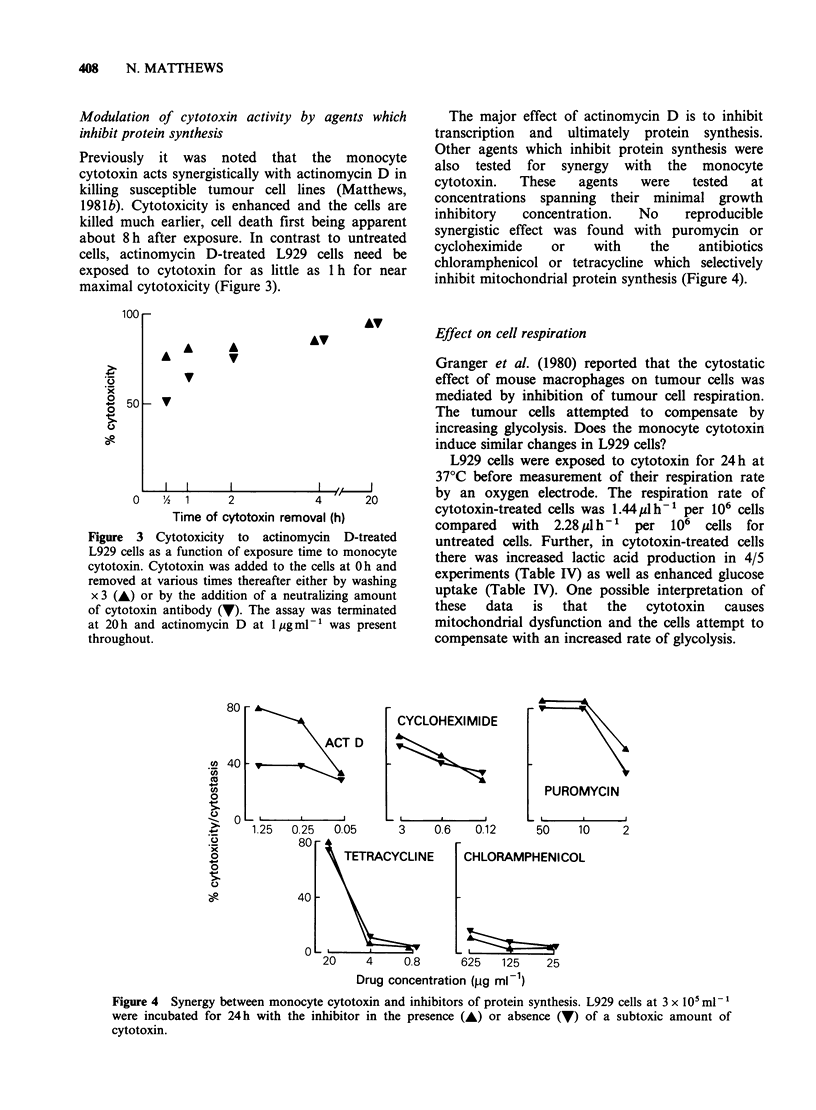
Human monocytes can be induced to synthesize a cytotoxin which affects certain tumour cell lines. The interaction of monocyte cytotoxin with a susceptible cell line (L929) has been studied to obtain clues to the mode of action of the cytotoxin. The cytotoxin acts directly on the cells rather than on the culture medium and is cytotoxic at higher concentrations and cytostatic at lower concentrations. First signs of cell damage appear about 20 h after contact with the cytotoxin which must be present throughout this period. The cytotoxin probably acts on the cell surface and is more effective at 40 degrees C than at 37 degrees C. For a given amount of cytotoxin the effects are inversely proportional to the target cell concentration. Treatment of the cytotoxin with phenanthroline inhibits cytotoxicity while treatment of the target cells with actinomycin D, but not cycloheximide or puromycin, enhances cytotoxicity. After 24 h cytotoxin treatment the target cells exhibit reduced respiration rate but enhanced glycolysis and glucose uptake suggesting mitochondrial dysfunction. A possible interpretation of these data is that the monocyte cytotoxin is a metalloenzyme which inactivates a cell surface receptor for a nutrient essential for mitochondrial function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Johnson W. J., Fiorito E., Nathan C. F. Hydrogen peroxide and cytolytic factor can interact synergistically in effecting cytolysis of neoplastic targets. J Immunol. 1981 Nov;127(5):1973–1977. [PubMed] [Google Scholar]
- Adams D. O., Kao K. J., Farb R., Pizzo S. V. Effector mechanisms of cytolytically activated macrophages. II. Secretion of a cytolytic factor by activated macrophages and its relationship to secreted neutral proteases. J Immunol. 1980 Jan;124(1):293–300. [PubMed] [Google Scholar]
- Aguet M. High-affinity binding of 125I-labelled mouse interferon to a specific cell surface receptor. Nature. 1980 Apr 3;284(5755):459–461. doi: 10.1038/284459a0. [DOI] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Matthews N. Human monocyte killing of tumor cells: contribution of an extracellular cytotoxin. Adv Exp Med Biol. 1982;155:721–729. doi: 10.1007/978-1-4684-4394-3_79. [DOI] [PubMed] [Google Scholar]
- Matthews N. Production of an anti-tumour cytotoxin by human monocytes. Immunology. 1981 Sep;44(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Matthews N. Production of an anti-tumour cytotoxin by human monocytes: comparison of endotoxin, interferons and other agents as inducers. Br J Cancer. 1982 Apr;45(4):615–617. doi: 10.1038/bjc.1982.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N. Tumour-necrosis factor from the rabbit. II. Production by monocytes. Br J Cancer. 1978 Aug;38(2):310–315. doi: 10.1038/bjc.1978.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N. Tumour-necrosis factor from the rabbit. V. Synthesis in vitro by mononuclear phagocytes from various tissues of normal and BCG-injected rabbits. Br J Cancer. 1981 Sep;44(3):418–424. doi: 10.1038/bjc.1981.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Watkins J. F. Tumour-necrosis factor from the rabbit. I. Mode of action, specificity and physicochemical properties. Br J Cancer. 1978 Aug;38(2):302–309. doi: 10.1038/bjc.1978.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Moore R. N., Mergenhagen S. E. Macrophages as a source of tumoricidal activity (tumor-necrotizing factor). Infect Immun. 1980 Nov;30(2):523–530. doi: 10.1128/iai.30.2.523-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen-Meyer J., Hammerstrøm J. Physicochemical characterization of cytostatic factors released from human monocytes. Infect Immun. 1982 Oct;38(1):67–73. doi: 10.1128/iai.38.1.67-73.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Rabbit tumor necrosis factor: mechanism of action. Infect Immun. 1981 Jan;31(1):380–385. doi: 10.1128/iai.31.1.380-385.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. K., Bramwell M. E., Harris H. Hexose transport in hybrids between malignant and normal cells. Nature. 1981 Nov 19;294(5838):232–235. doi: 10.1038/294232a0. [DOI] [PubMed] [Google Scholar]


