Abstract
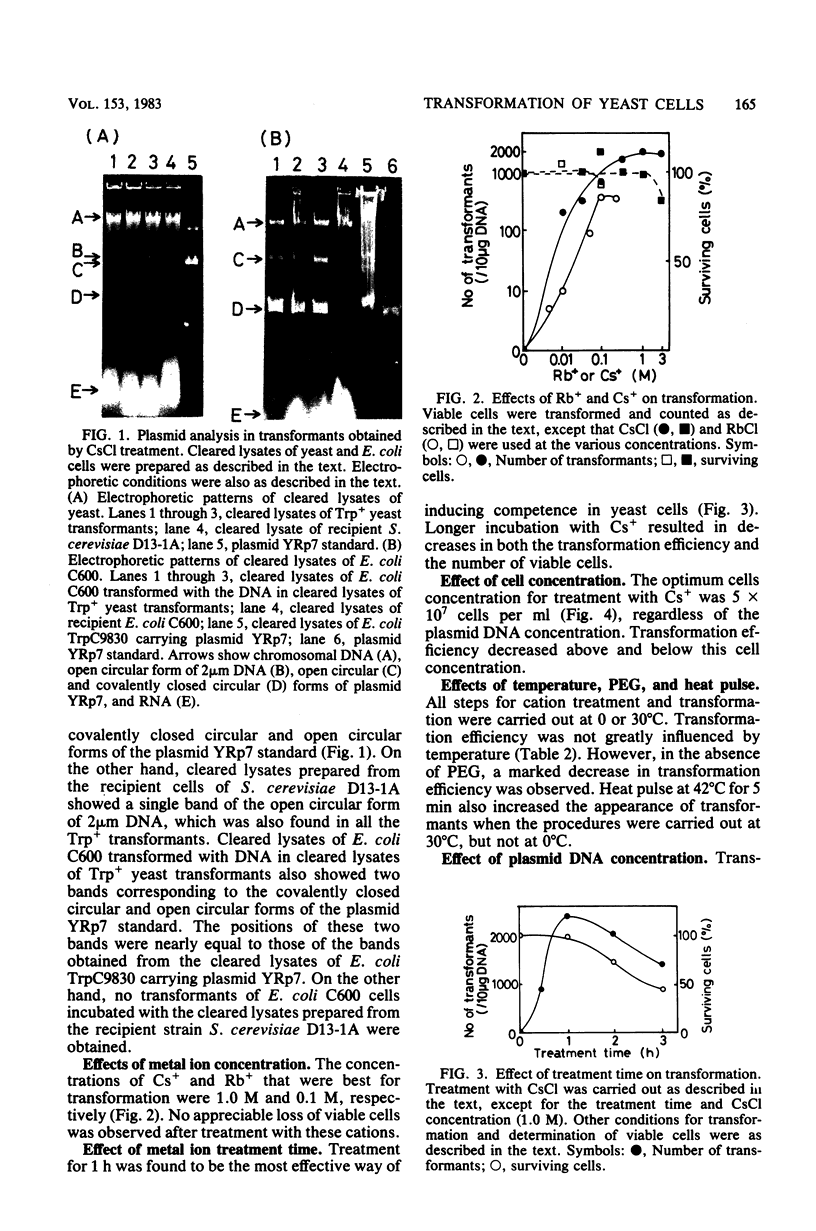
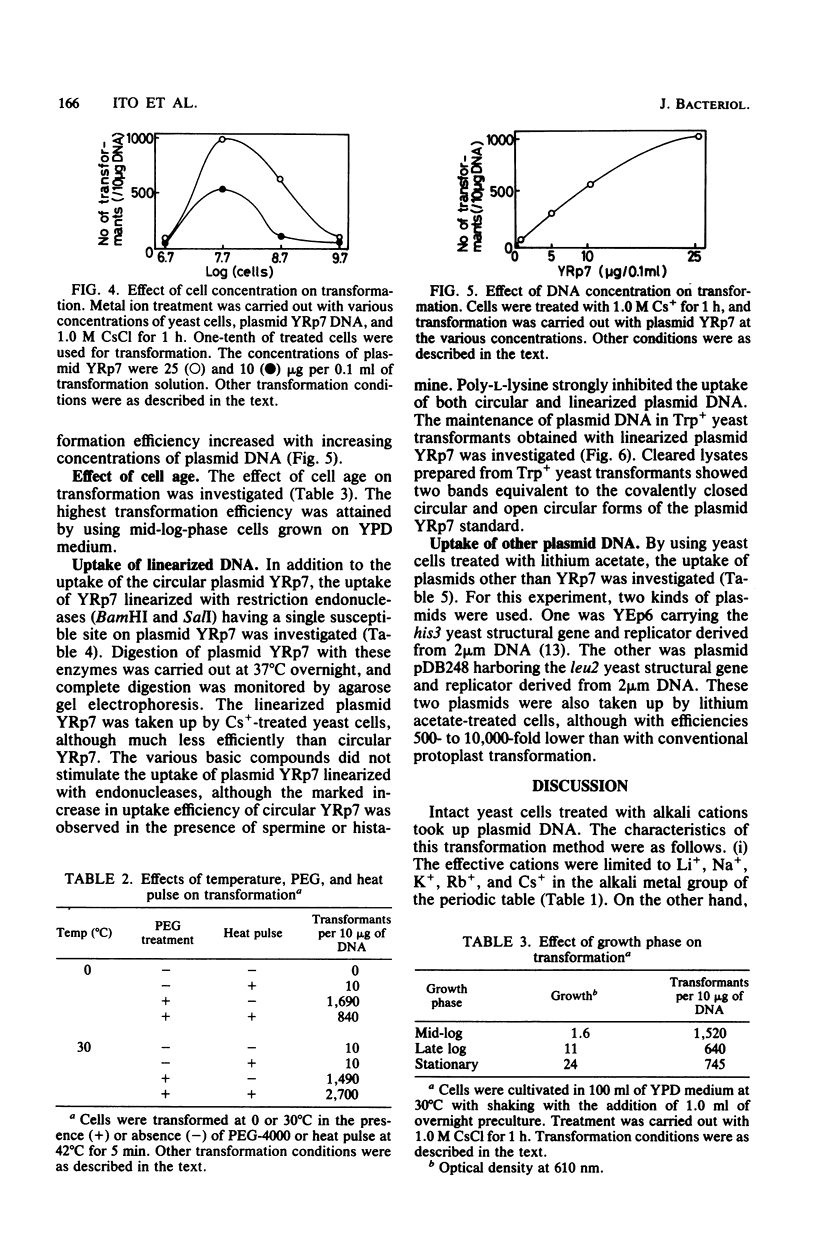
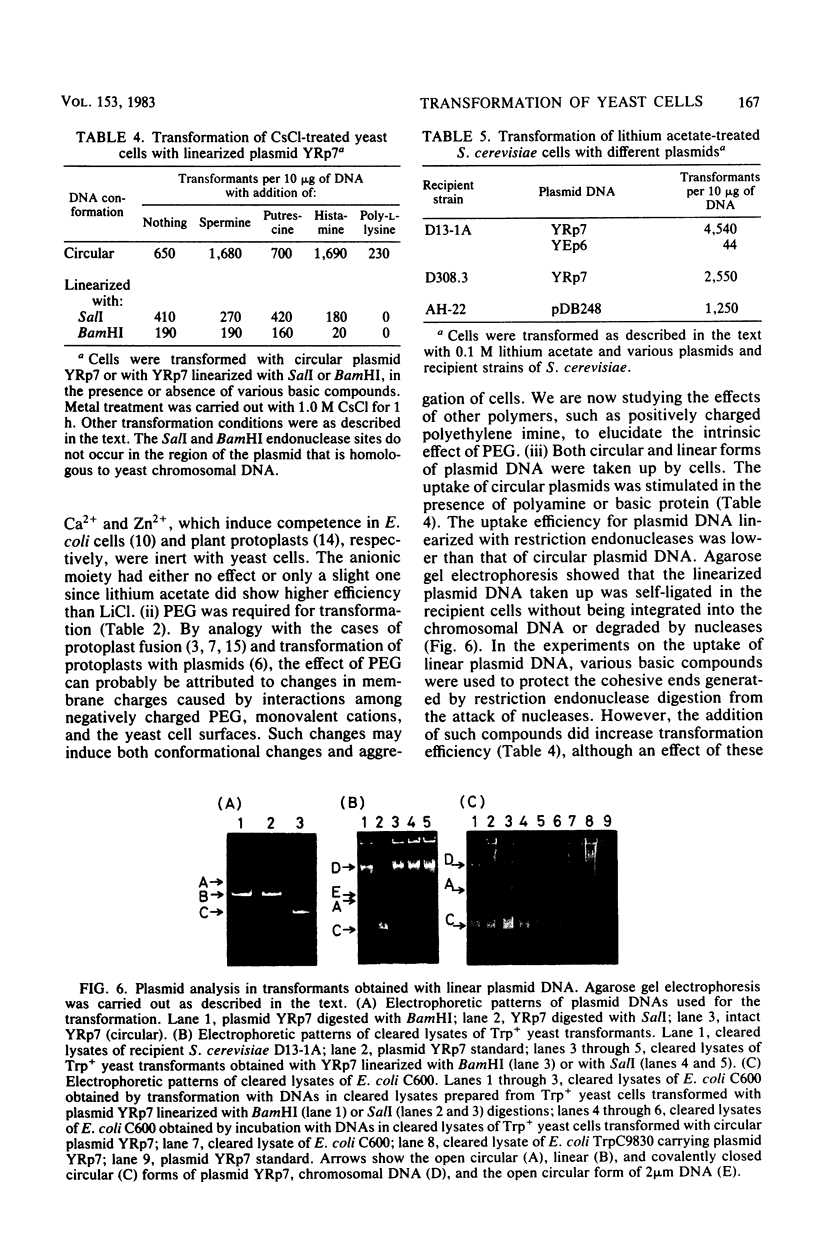
Intact yeast cells treated with alkali cations took up plasmid DNA. Li+, Cs+, Rb+, K+, and Na+ were effective in inducing competence. Conditions for the transformation of Saccharomyces cerevisiae D13-1A with plasmid YRp7 were studied in detail with CsCl. The optimum incubation time was 1 h, and the optimum cell concentration was 5 x 10(7) cells per ml. The optimum concentration of Cs+ was 1.0 M. Transformation efficiency increased with increasing concentrations of plasmid DNA. Polyethylene glycol was absolutely required. Heat pulse and various polyamines or basic proteins stimulated the uptake of plasmid DNA. Besides circular DNA, linear plasmid DNA was also taken up by Cs+-treated yeast cells, although the uptake efficiency was considerably reduced. The transformation efficiency with Cs+ or Li+ was comparable with that of conventional protoplast methods for a plasmid containing ars1, although not for plasmids containing a 2 microns origin replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron J. R., Philippsen P., Davis R. W. Analysis of chromosomal integration and deletions of yeast plasmids. Nucleic Acids Res. 1977;4(5):1429–1448. doi: 10.1093/nar/4.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczy L., Maráz A. Transfer of mitochondria by protoplast fusion in Saccharomyces cerevisiae. Nature. 1977 Aug 11;268(5620):524–525. doi: 10.1038/268524a0. [DOI] [PubMed] [Google Scholar]
- Grinius L. Nucleic acid transport driven by ion gradient across cell membrane. FEBS Lett. 1980 Apr 21;113(1):1–10. doi: 10.1016/0014-5793(80)80482-0. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Horn V., Bonner M., Stasiowski S. Polarity and enzyme functions in mutants of the first three genes of the tryptophan operon of Escherichia coli. Genetics. 1971 Dec;69(4):409–433. doi: 10.1093/genetics/69.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Solingen P., van der Plaat J. B. Fusion of yeast spheroplasts. J Bacteriol. 1977 May;130(2):946–947. doi: 10.1128/jb.130.2.946-947.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]