Full text
PDF
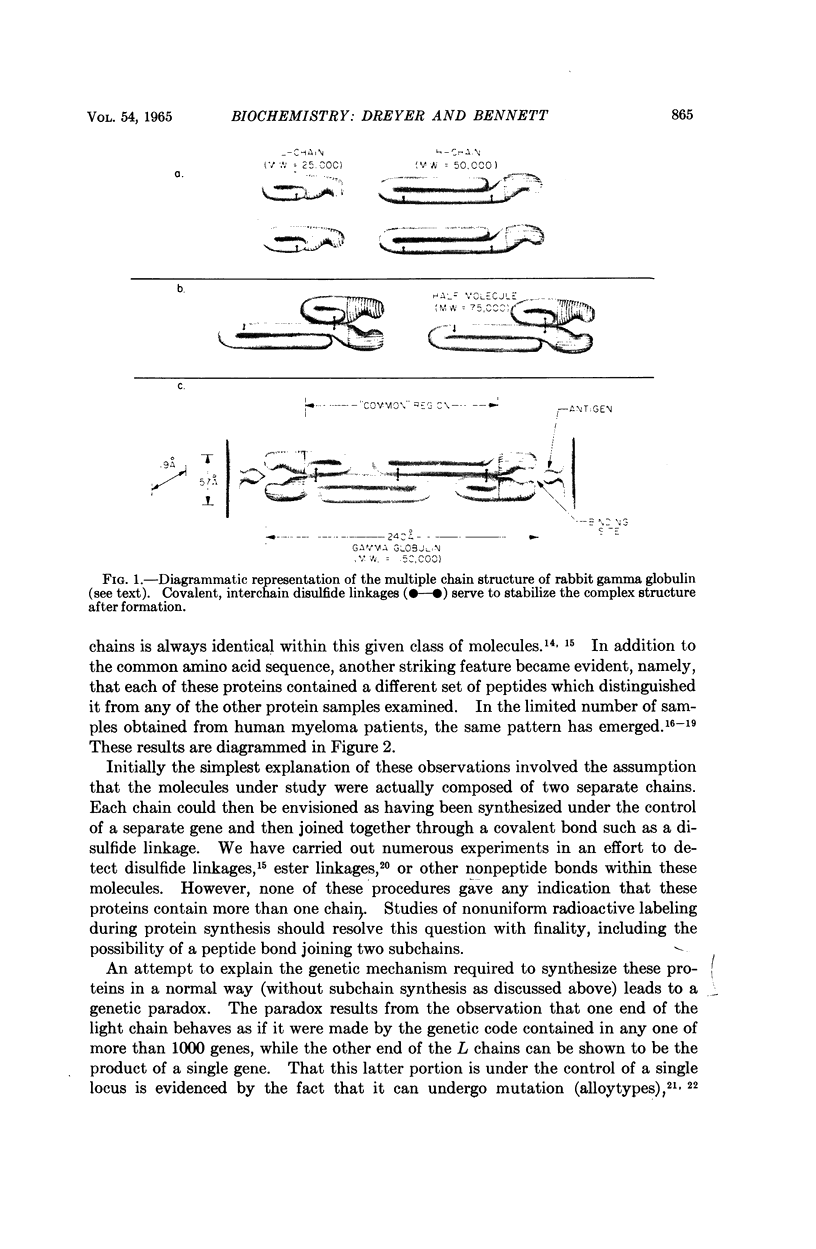
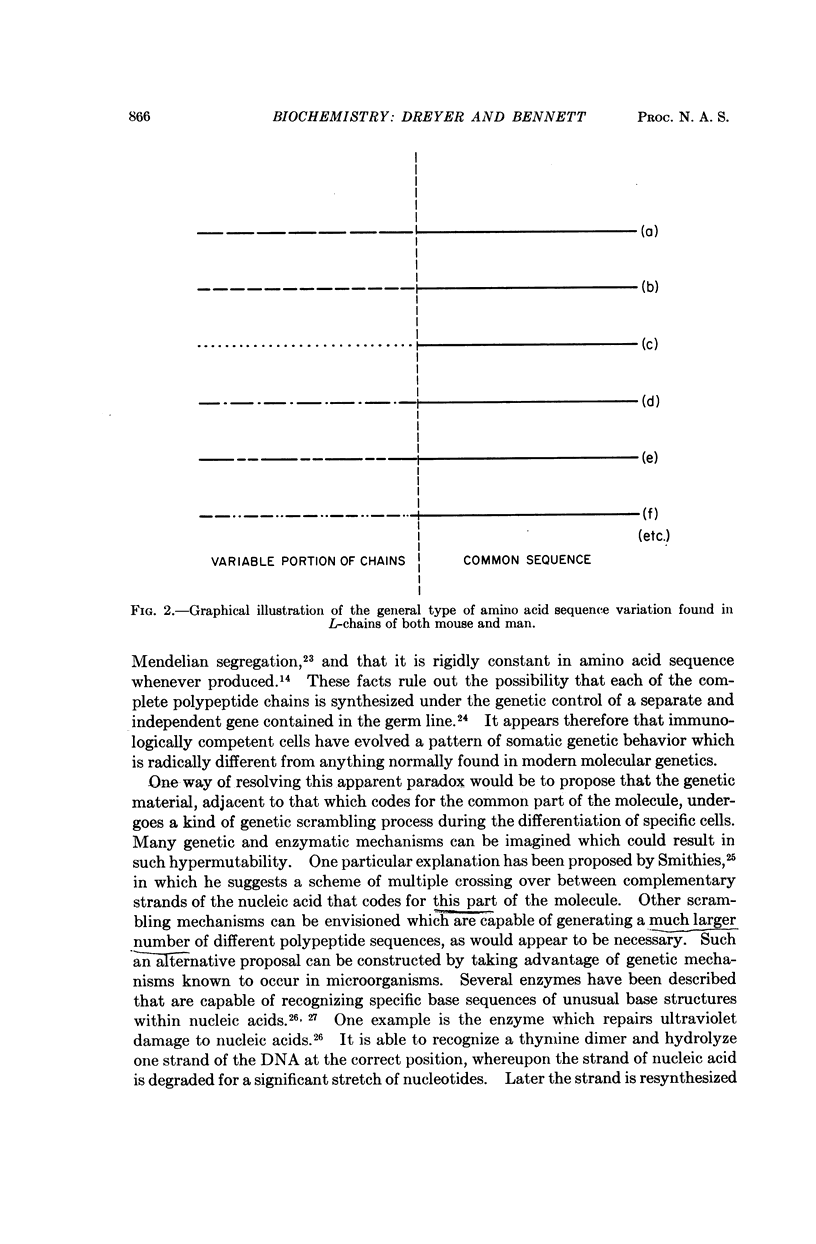
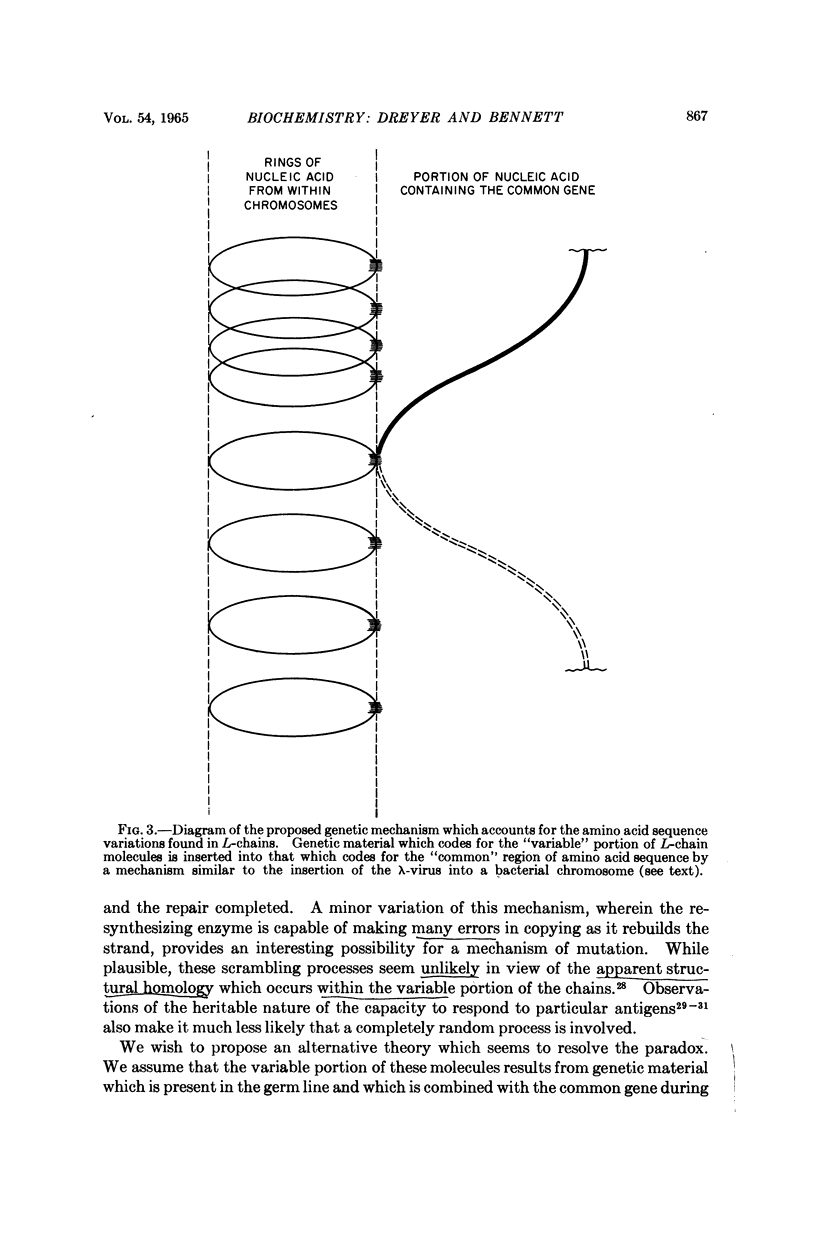


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALMEIDA J., CINADER B., HOWATSON A. THE STRUCTURE OF ANTIGEN-ANTIBODY COMPLEXES. A STUDY BY ELECTRON MICROSCOPY. J Exp Med. 1963 Sep 1;118:327–340. doi: 10.1084/jem.118.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT J. C. GENETIC CODING FOR PROTEIN STRUCTURE. Annu Rev Biochem. 1964;33:205–234. doi: 10.1146/annurev.bi.33.070164.001225. [DOI] [PubMed] [Google Scholar]
- BENNETT J. C., HOOD L. E., DREYER W. J., POTTER M. EVIDENCE FOR AMINO ACID SEQUENCE DIFFERENCES AMONG PROTEINS RESEMBLING THE L-CHAIN SUBUNITS OF IMMUNOGLOBULINS. J Mol Biol. 1965 May;12:81–87. doi: 10.1016/s0022-2836(65)80284-4. [DOI] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- EDELMAN G. M., GALLY J. A. A MODEL FOR THE 7S ANTIBODY MOLECULE. Proc Natl Acad Sci U S A. 1964 May;51:846–853. doi: 10.1073/pnas.51.5.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN G. M., GALLY J. A. The nature of Bence-Jones proteins. Chemical similarities to polypetide chains of myeloma globulins and normal gamma-globulins. J Exp Med. 1962 Aug 1;116:207–227. doi: 10.1084/jem.116.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIERS W., SINSHEIMER R. L. The structure of the DNA of bacteriophage phi-X174. III. Ultracentrifugal evidence for a ring structure. J Mol Biol. 1962 Oct;5:424–434. doi: 10.1016/s0022-2836(62)80031-x. [DOI] [PubMed] [Google Scholar]
- FINSTAD J., PAPERMASTER B. W., GOOD R. A. EVOLUTION OF THE IMMUNE RESPONSE. II. MORPHOLOGIC STUDIES ON THE ORIGIN OF THE THYMUS AND ORGANIZED LYMPHOID TISSUE. Lab Invest. 1964 May;13:490–512. [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- GOLD M., HAUSMANN R., MAITRA U., HURWITZ J. THE ENZYMATIC METHYLATION OF RNA AND DNA. 8. EFFECTS OF BACTERIOPHAGE INFECTION ON THE ACTIVITY OF THE METHYLATING ENZYMES. Proc Natl Acad Sci U S A. 1964 Aug;52:292–297. doi: 10.1073/pnas.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABER E. RECOVERY OF ANTIGENIC SPECIFICITY AFTER DENATURATION AND COMPLETE REDUCTION OF DISULFIDES IN A PAPAIN FRAGMENT OF ANTIBODY. Proc Natl Acad Sci U S A. 1964 Oct;52:1099–1106. doi: 10.1073/pnas.52.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTTA Y., BASSEL A. MOLECULAR SIZE AND CIRCULARITY OF DNA IN CELLS OF MAMMALS AND HIGHER PLANTS. Proc Natl Acad Sci U S A. 1965 Feb;53:356–362. doi: 10.1073/pnas.53.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Sur les processus de conjugaison et de recombinaison génétique chez Escherichia coli. IV. Prophages inductibles et mesure des segments génétiques transferés au cours de la conjugaison. Ann Inst Pasteur (Paris) 1958 Nov;95(5):497–519. [PubMed] [Google Scholar]
- LAWLER S. D., COHEN S. DISTRIBUTION OF ALLOTYPIC SPECIFICITIES ON THE PEPTIDE CHAINS OF HUMAN GAMMA-GLOBULIN. Immunology. 1965 Feb;8:206–212. [PMC free article] [PubMed] [Google Scholar]
- MCCLINTOCK B. Controlling elements and the gene. Cold Spring Harb Symp Quant Biol. 1956;21:197–216. doi: 10.1101/sqb.1956.021.01.017. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., DIXON D. J. EVIDENCE FOR LINKAGE OF UNIVALENT FRAGMENTS OR HALF-MOLECULES OF RABBIT GAMMA-GLOBULIN BY THE SAME DISULFIDE BOND. Biochemistry. 1964 Sep;3:1338–1342. doi: 10.1021/bi00897a025. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- PAIN R. H. THE MOLECULAR WEIGHTS OF THE PEPTIDE CHAINS OF GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:234–239. doi: 10.1042/bj0880234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPERMASTER B. W., CONDIE R. M., FINSTAD J., GOOD R. A. EVOLUTION OF THE IMMUNE RESPONSE. I. THE PHYLOGENETIC DEVELOPMENT OF ADAPTIVE IMMUNOLOGIC RESPONSIVENESS IN VERTEBRATES. J Exp Med. 1964 Jan 1;119:105–130. doi: 10.1084/jem.119.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON R. D., COOPER M. D., GOOD R. A. THE PATHOGENESIS OF IMMUNOLOGIC DEFICIENCY DISEASES. Am J Med. 1965 Apr;38:579–604. doi: 10.1016/0002-9343(65)90135-x. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTTER M., DREYER W. J., KUFF E. L., MCINTIRE K. R. HERITABLE VARIATION IN BENCE JONES PROTEIN STRUCTURE IN AN INBRED STRAIN OF MICE. J Mol Biol. 1964 Jun;8:814–822. doi: 10.1016/s0022-2836(64)80162-5. [DOI] [PubMed] [Google Scholar]
- PTASHNE M. THE DETACHMENT AND MATURATION OF CONSERVED LAMBDA PROPHAGE DNA. J Mol Biol. 1965 Jan;11:90–96. doi: 10.1016/s0022-2836(65)80174-7. [DOI] [PubMed] [Google Scholar]
- PUTNAM F. W., EASLEY C. W., HELLING J. W. STRUCTURAL STUDY OF HUMAN GAMMA-GLOBULIN THROUGH THE ANALYSIS OF THE TRYPTIC PEPTIDES OF BENCE-JONES PROTEINS. Biochim Biophys Acta. 1963 Oct 8;78:231–233. doi: 10.1016/0006-3002(63)91632-9. [DOI] [PubMed] [Google Scholar]
- PUTNAM F. W., EASLEY C. W. STRUCTURAL STUDIES OF THE IMMUNOGLOBULINS. I. THE TRYPTIC PEPTIDES OF BENCE-JONES PROTEINS. J Biol Chem. 1965 Apr;240:1626–1638. [PubMed] [Google Scholar]
- ROPARTZ C., LENOIR J., RIVAT L. A new inheritable property of human sera: the InV factor. Nature. 1961 Feb 18;189:586–586. doi: 10.1038/189586a0. [DOI] [PubMed] [Google Scholar]
- SMALL P. A., Jr, KEHN J. E., LAMM M. E. POLYPEPTIDE CHAINS OF RABBIT GAMMAGLOBULIN. Science. 1963 Oct 18;142(3590):393–394. doi: 10.1126/science.142.3590.393. [DOI] [PubMed] [Google Scholar]
- SMALL P. A., Jr, REISFELD R. A., DRAY S. PEPTIDE DIFFERENCES OF RABBIT GAMMA-G-IMMUNOGLOBULIN LIGHT CHAINS CONTROLLED BY ALLELIC GENES. J Mol Biol. 1965 Apr;11:713–721. doi: 10.1016/s0022-2836(65)80029-8. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. GAMMA-GLOBULIN VARIABILITY: A GENETIC HYPOTHESIS. Nature. 1963 Sep 28;199:1231–1236. doi: 10.1038/1991231a0. [DOI] [PubMed] [Google Scholar]
- TITANI K., PUTNAM F. W. IMMUNOGLOBULIN STRUCTURE: AMINO- AND CARBOXYL-TERMINAL PEPTIDES OF TYPE I BENCE JONES PROTEINS. Science. 1965 Mar 12;147(3663):1304–1305. doi: 10.1126/science.147.3663.1304. [DOI] [PubMed] [Google Scholar]
- URSO P., MAKINODAN T. THE ROLES OF CELLULAR DIVISION AND MATURATION IN THE FORMATION OF PRECIPITATING ANTIBODY. J Immunol. 1963 Jun;90:897–907. [PubMed] [Google Scholar]
- VAN EIJKH, MONFOORT C. H., WESTENBRINK H. G. PURIFICATION, ANALYSIS AND PROPERTIES OF SOME BENCE-JONES PROTEINS. Proc K Ned Akad Wet C. 1963;66:345–362. [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITNEY P. L., TANFORD C. RECOVERY OF SPECIFIC ACTIVITY AFTER COMPLETE UNFOLDING AND REDUCTION OF AN ANTIBODY FRAGMENT. Proc Natl Acad Sci U S A. 1965 Mar;53:524–532. doi: 10.1073/pnas.53.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]





