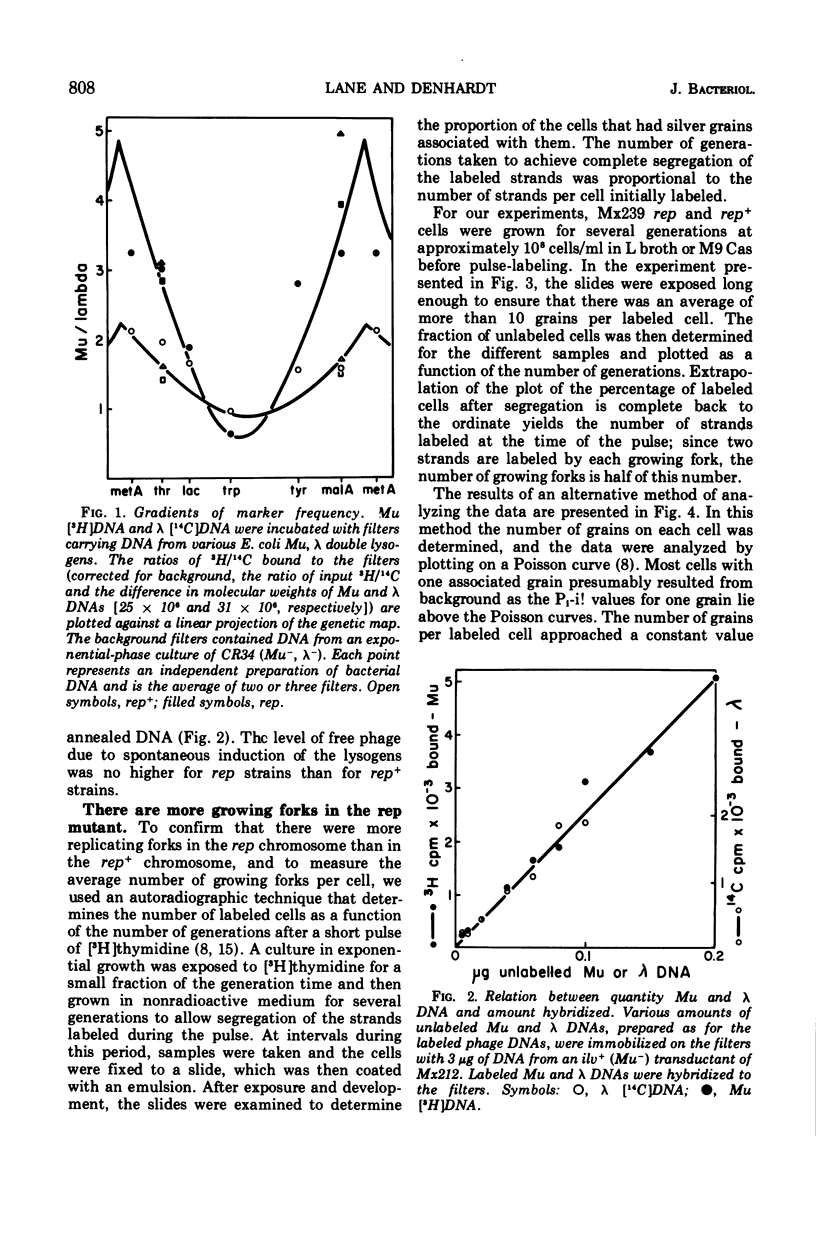
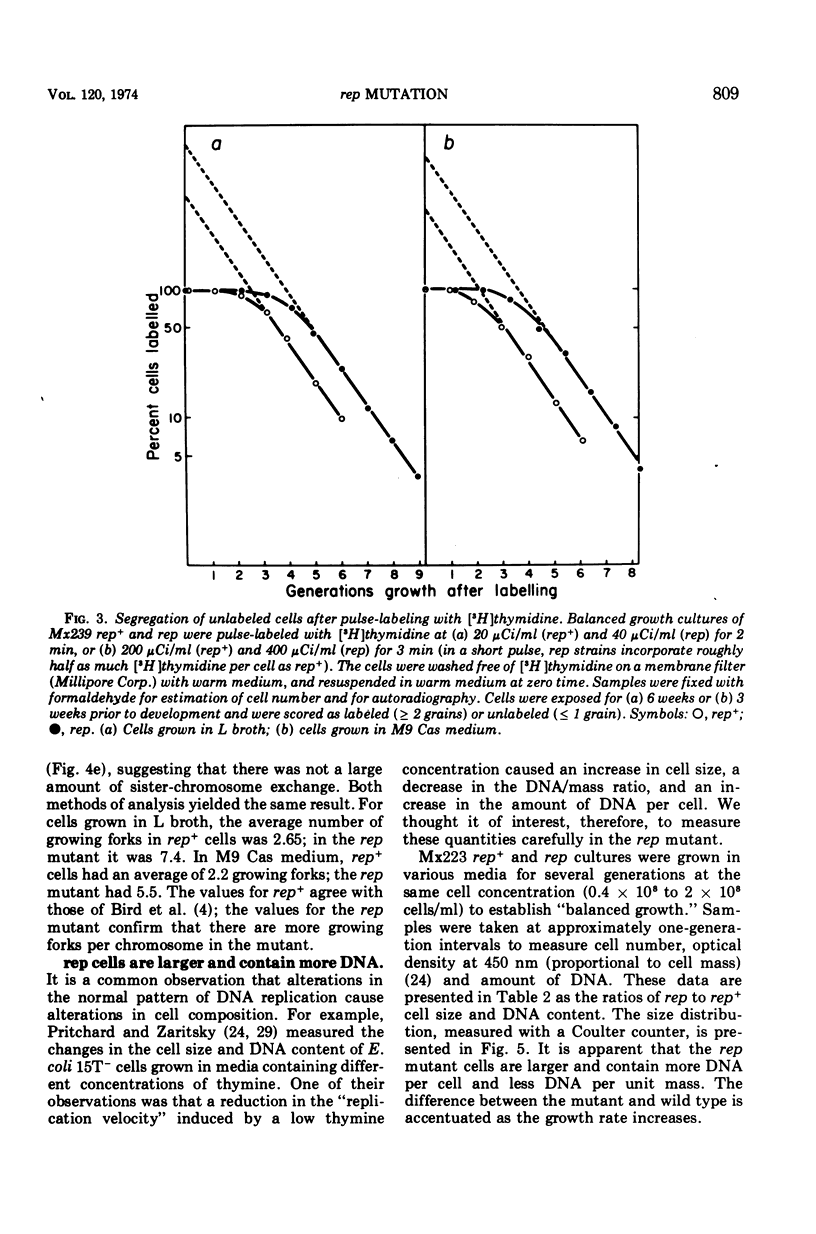
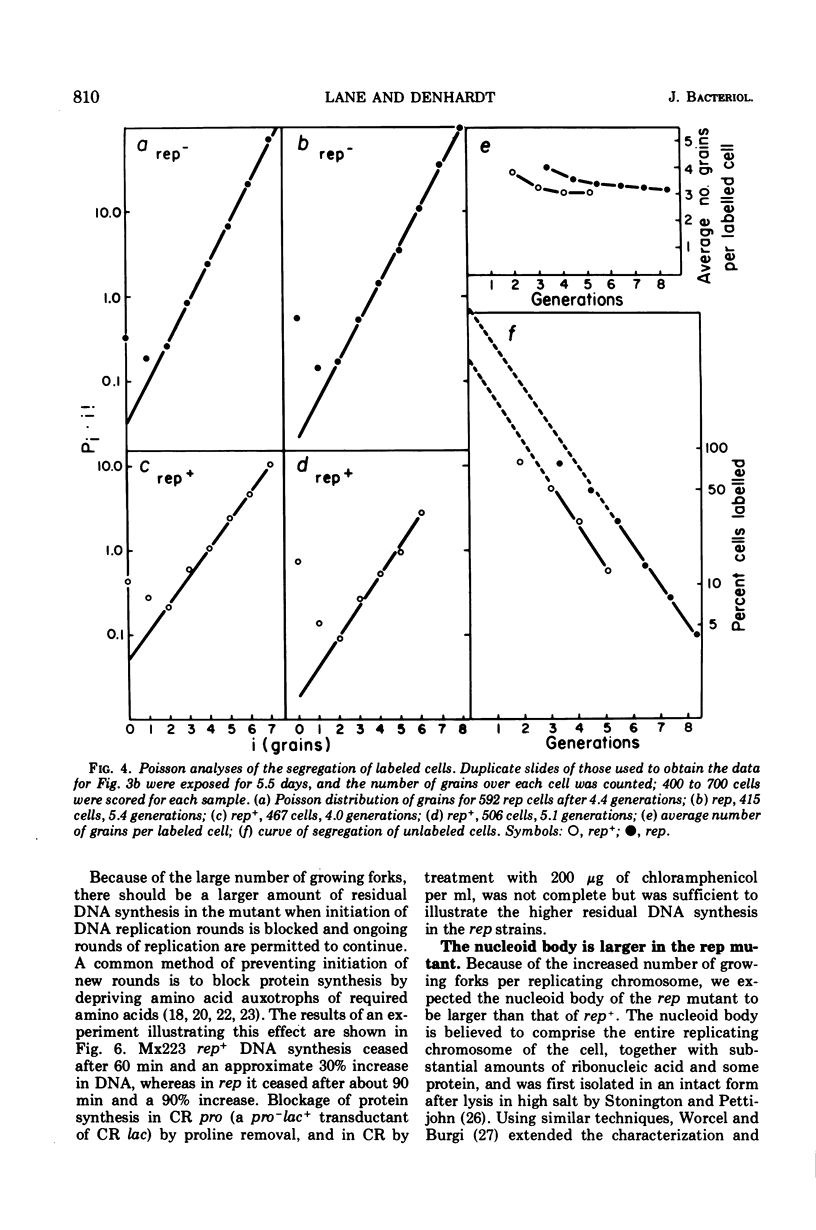
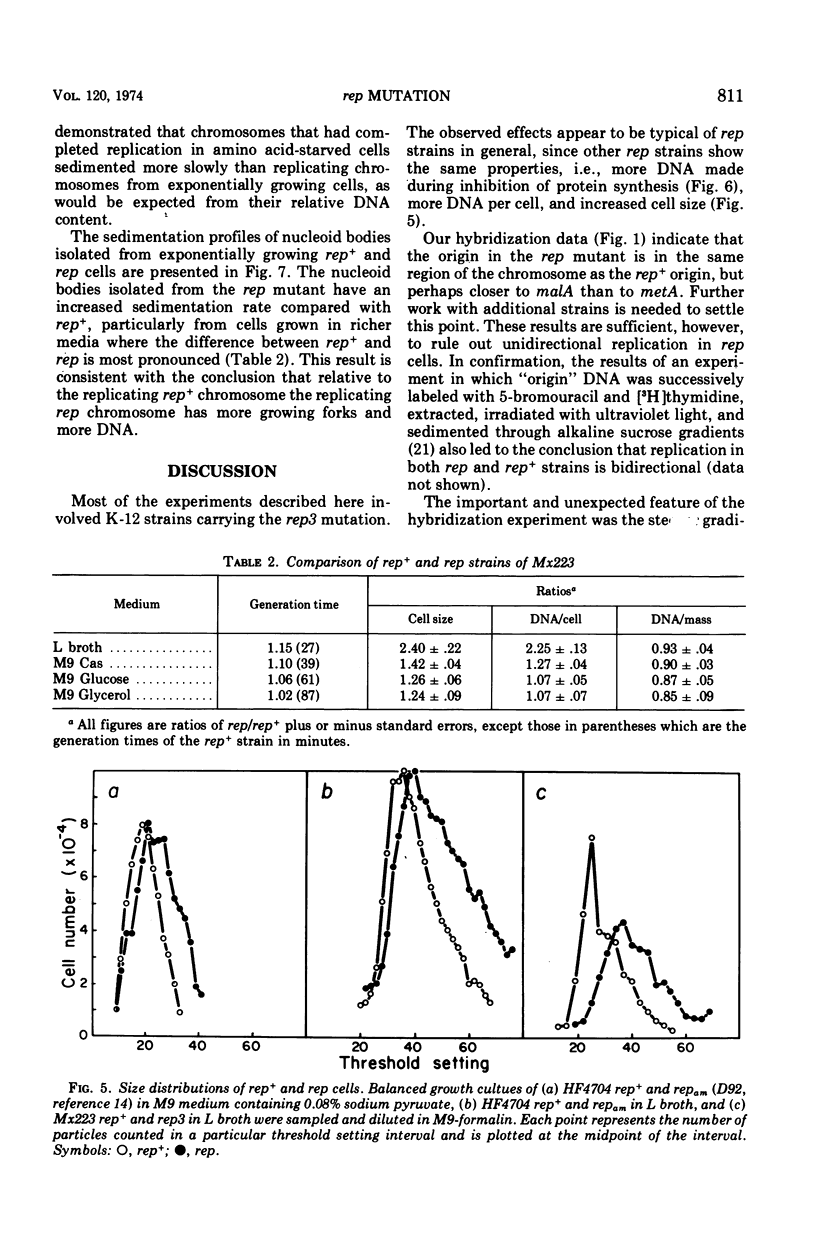
Abstract
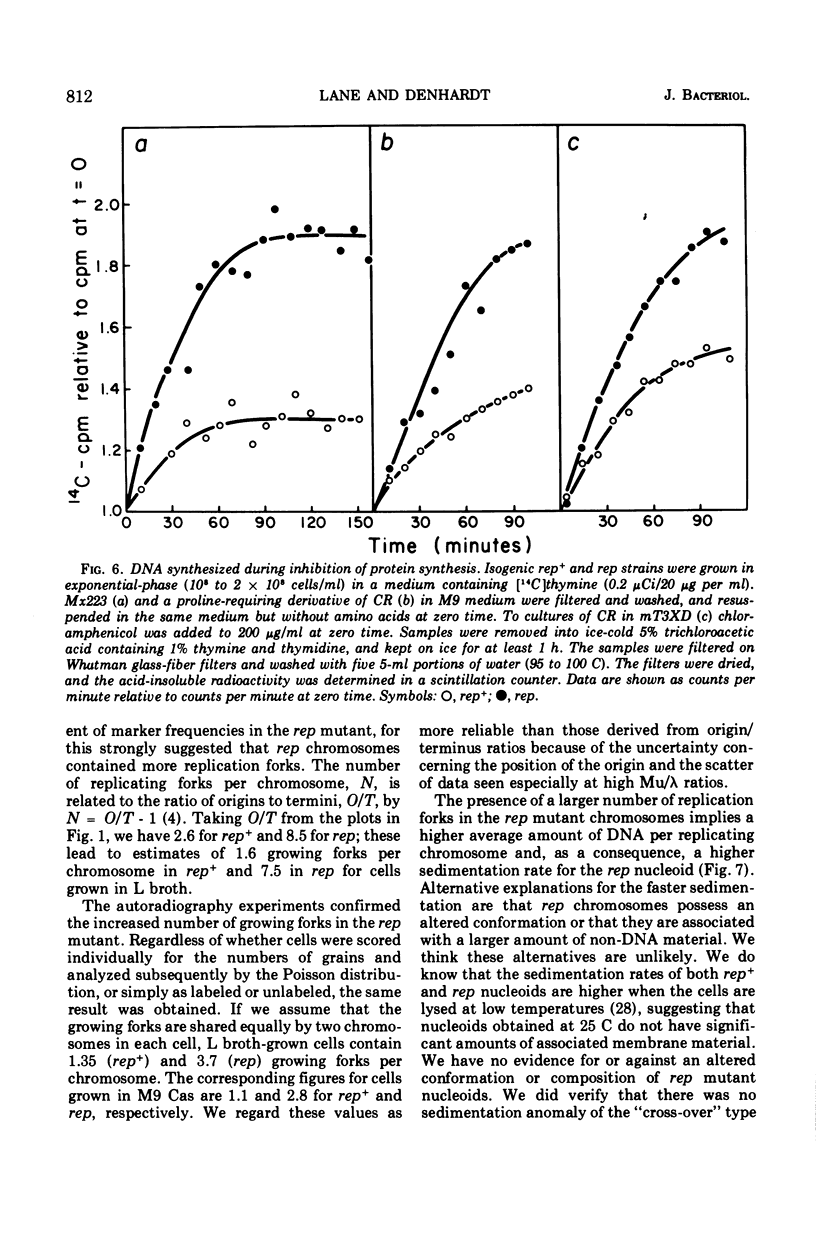
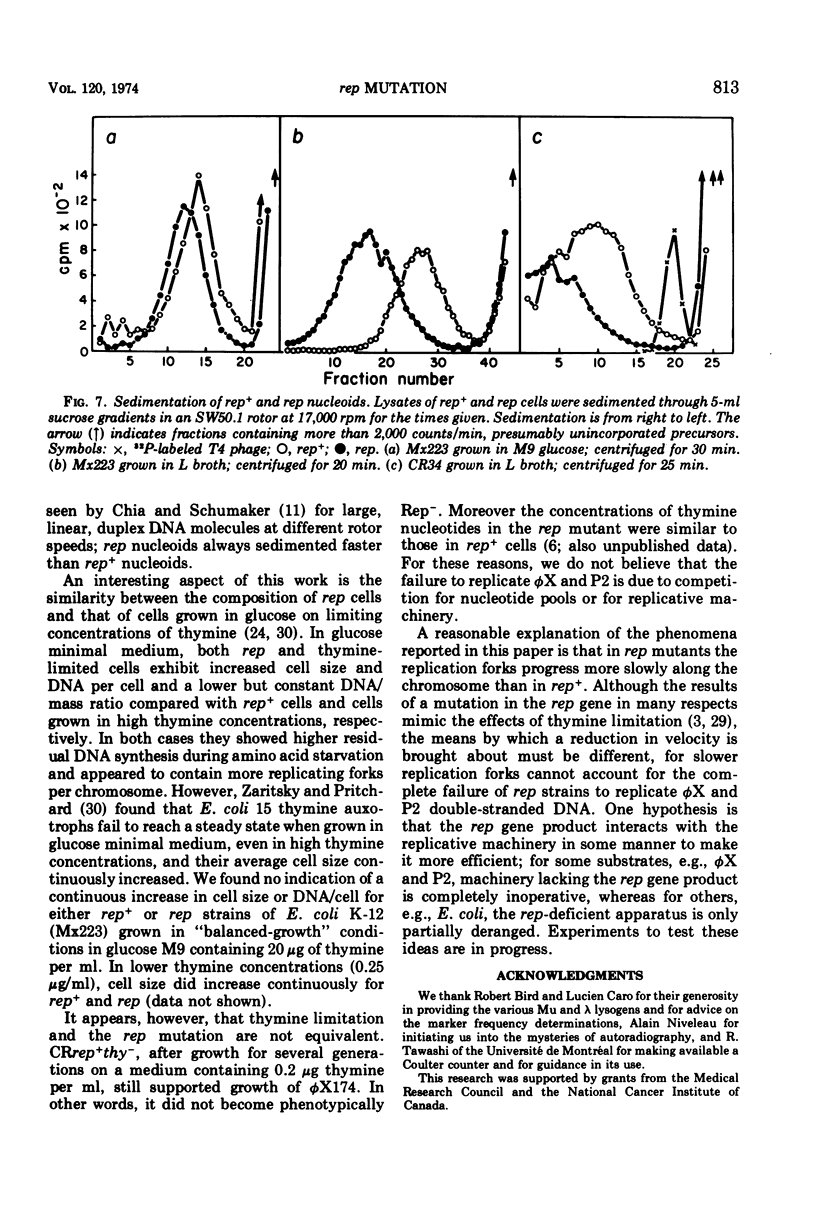
The rep gene function of Escherichia coli is essential for the replication of P2 and φX174 double-stranded deoxyribonucleic acid (DNA). Compared with isogenic rep+ strains, rep mutants show the following characteristics: larger cell size, more DNA per cell, and a slightly lower DNA/mass ratio. The replicating rep chromosomes show a steeper gradient of marker frequencies and contain more replicating forks per chromosome. The nucleoid body of rep mutants sediments faster and contains more DNA. We deduce that the rep function is required for the “normal” replication of the E. coli chromosome and that in its absence the E. coli chromosome replicates in an altered manner, perhaps involving slower-moving replicating forks.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. D., Jansz H. S. PhiX174 replicative form DNA replication, origin and direction. J Mol Biol. 1972 Feb 14;63(3):569–576. doi: 10.1016/0022-2836(72)90448-2. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Beacham K., Zaritsky A., Pritchard R. H. Intracellular thymidine triphosphate concentrations in wild type and in thymine requiring mutants of Escherichia coli 15 and K12. J Mol Biol. 1971 Aug 28;60(1):75–86. doi: 10.1016/0022-2836(71)90448-7. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Louarn J., Martuscelli J., Caro L. Origin and sequence of chromosome replication in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):549–566. doi: 10.1016/0022-2836(72)90559-1. [DOI] [PubMed] [Google Scholar]
- Calendar R., Lindqvist B., Sironi G., Clark A. J. Characterization of REP- mutants and their interaction with P2 phage. Virology. 1970 Jan;40(1):72–83. doi: 10.1016/0042-6822(70)90380-6. [DOI] [PubMed] [Google Scholar]
- Caro L. G., Berg C. M. Chromosome replication in some strains of Escherichia coli K12. Cold Spring Harb Symp Quant Biol. 1968;33:559–573. doi: 10.1101/sqb.1968.033.01.063. [DOI] [PubMed] [Google Scholar]
- Caro L. G. Chromosome replication in Escherichia coli. 3. Segregation of chromosomal strands in multi-forked replication. J Mol Biol. 1970 Mar 14;48(2):329–338. doi: 10.1016/0022-2836(70)90164-6. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Inman R. B. Origin and direction of replication of bacteriophage 186 DNA. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1768–1771. doi: 10.1073/pnas.70.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia D., Schumaker V. N. A rotor speed dependent crossover in sedimentation velocities of DNA's of different sizes. Biochem Biophys Res Commun. 1974 Jan;56(1):241–246. doi: 10.1016/s0006-291x(74)80340-2. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Dressler D. H., Hathaway A. THE ABORTIVE REPLICATION OF PhiX174 DNA IN A RECOMBINATION-DEFICIENT MUTANT OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):813–820. doi: 10.1073/pnas.57.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. Formation of ribosylthymine in Escherichia coli. Studies on pulse labeling with thymine and thymidine. J Biol Chem. 1969 May 25;244(10):2710–2715. [PubMed] [Google Scholar]
- Denhardt D. T., Iwaya M., Larison L. L. The rep mutation. II. Its effect on Escherichia coli and on the replication of bacteriophage phi X174. Virology. 1972 Aug;49(2):486–496. doi: 10.1016/0042-6822(72)90500-4. [DOI] [PubMed] [Google Scholar]
- Eberle H., Lark K. G. Chromosome replication in Bacillus subtilis cultures growing at different rates. Proc Natl Acad Sci U S A. 1967 Jan;57(1):95–101. doi: 10.1073/pnas.57.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi T., Nagata T. Mutations affecting growth of the Escherichia coli cell under a condition of DNA polymerase I-deficiency. Mol Gen Genet. 1973;123(1):89–110. doi: 10.1007/BF00282992. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Leidner J., Tremblay G. Y. DNA-DNA hybridization on filters at low temperature in the presence of formamide or urea. Biochimie. 1971;53(10):1111–1114. doi: 10.1016/s0300-9084(71)80201-8. [DOI] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Marunouchi T., Messer W. Replication of a specific terminal chromosome segment in Escherichia coli which is required for cell division. J Mol Biol. 1973 Jun 25;78(1):211–228. doi: 10.1016/0022-2836(73)90439-7. [DOI] [PubMed] [Google Scholar]
- McKenna W. G., Masters M. Biochemical evidence for the bidirectional replication of DNA in Escherichia coli. Nature. 1972 Dec 29;240(5383):536–539. doi: 10.1038/240536a0. [DOI] [PubMed] [Google Scholar]
- PRITCHARD R. H., LARK K. G. INDUCTION OF REPLICATION BY THYMINE STARVATION AT THE CHROMOSOME ORIGIN IN ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:288–307. doi: 10.1016/s0022-2836(64)80208-4. [DOI] [PubMed] [Google Scholar]
- Pritchard R. H., Zaritsky A. Effect of thymine concentration on the replication velocity of DNA in a thymineless mutant of Escherichia coli. Nature. 1970 Apr 11;226(5241):126–131. doi: 10.1038/226126a0. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Starting point and direction of replication in P2 DNA. J Mol Biol. 1971 Jan 14;55(1):31–38. doi: 10.1016/0022-2836(71)90278-6. [DOI] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. doi: 10.1016/0022-2836(74)90576-2. [DOI] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Changes in cell size and shape associated with changes in the replication time of the chromosome of Escherichia coli. J Bacteriol. 1973 May;114(2):824–837. doi: 10.1128/jb.114.2.824-837.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Replication time of the chromosome in thymineless mutants of Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):65–74. doi: 10.1016/0022-2836(71)90447-5. [DOI] [PubMed] [Google Scholar]


