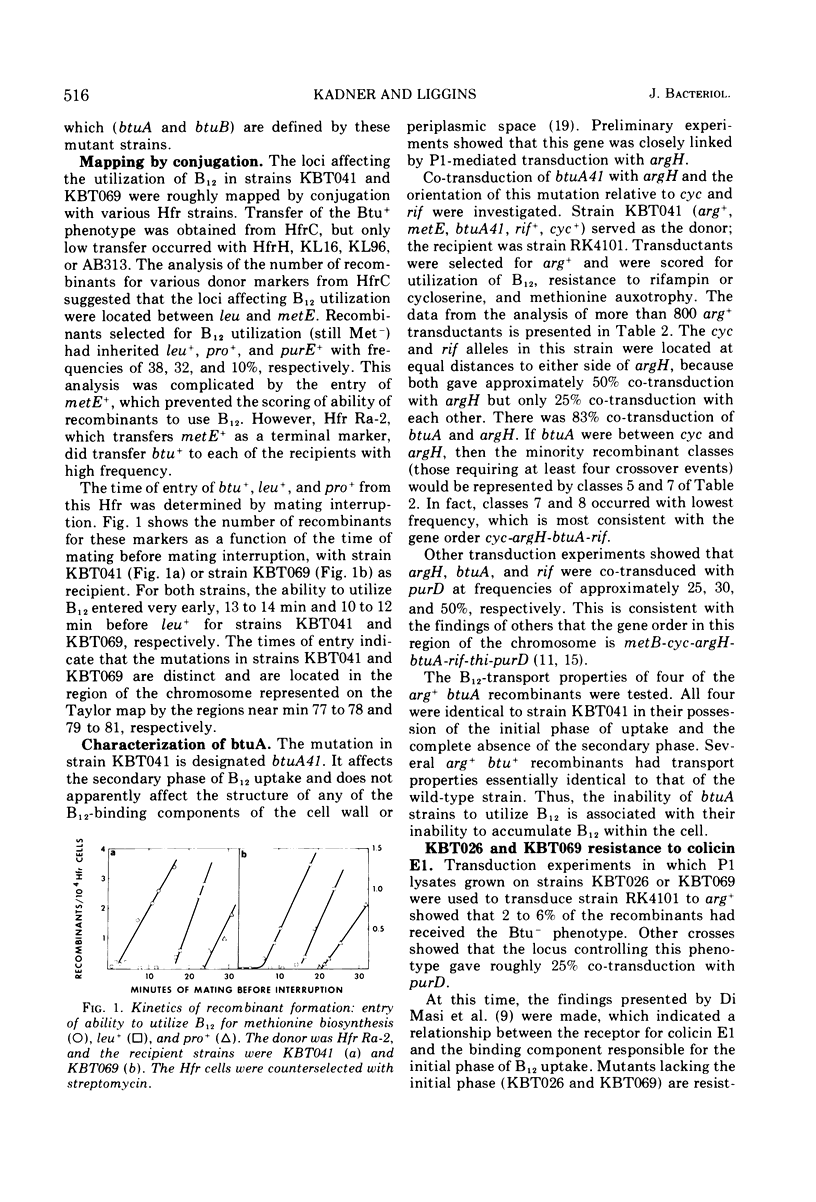
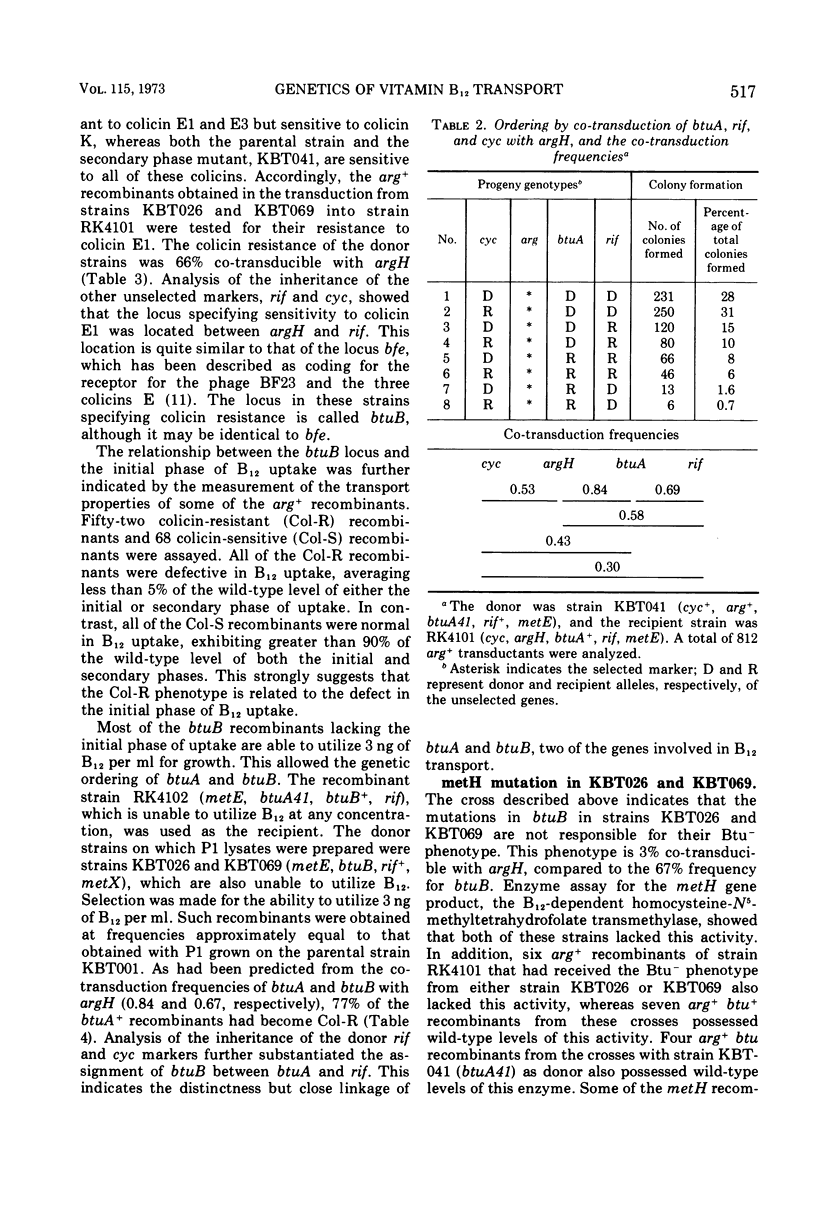
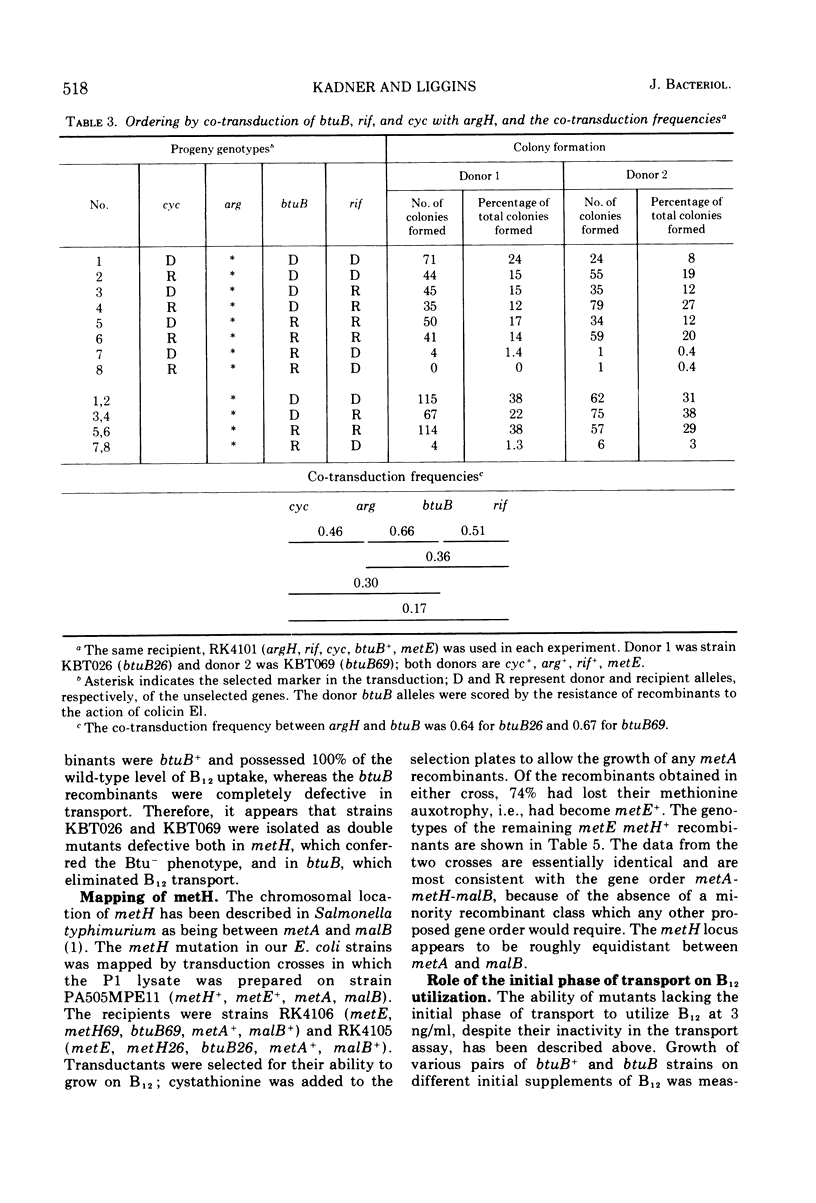
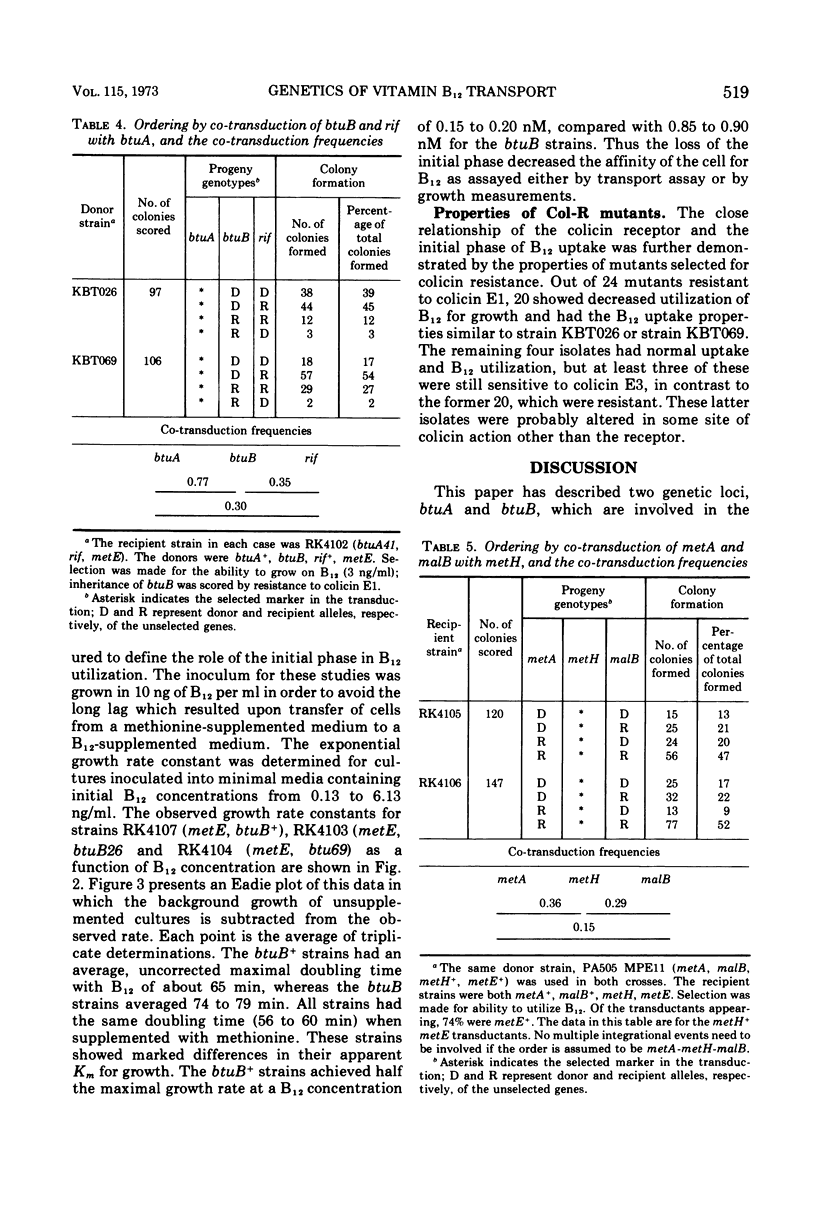
Abstract
The chromosomal location of two genetic loci involved in the transport of cyanocobalamin (B12) in Escherichia coli K-12 was determined. One gene, btuA, is believed to code for the transport protein in the cytoplasmic membrane, because a mutant with an alteration in this gene has lost the ability to accumulate B12 within the cell although normal levels of the surface receptors for B12 are present. The other locus, btuB, apparently codes for the surface receptor on the outer membrane. These mutants have lost the ability to bind B12 and have greatly reduced transport activity, although growth experiments have shown that they can utilize B12 for growth, but with decreased efficiency. This surface receptor for B12 also appears to function as the receptor for the E colicins, because btuB mutants are resistant to the E colicins, and mutants selected for resistance to colicin E1 are defective in B12 binding and transport. The gene order was determined by transduction analysis to be cyc-argH-btuA-btuB-rif-purD. In addition, mutations in metH, the gene for the B12-dependent homocysteine methylating enzyme, were obtained in this study. This gene was localized between metA and malB.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayling P. D., Chater K. F. The sequence of four structural and two regulatory methionine genes in the Salmonella typhimurium linkage map. Genet Res. 1968 Dec;12(3):341–354. doi: 10.1017/s0016672300011927. [DOI] [PubMed] [Google Scholar]
- Campbell A., Del Campillo-Campbell A., Chang R. A mutant of Escherichia coli that requires high concentrations of biotin. Proc Natl Acad Sci U S A. 1972 Mar;69(3):676–680. doi: 10.1073/pnas.69.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Caro L. G., Allison D. P., Stallions D. R. Early stages of conjugation in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1091–1104. doi: 10.1128/jb.100.2.1091-1104.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Berg C. M., Harris P. E. Kinetic and genetic analyses of D-cycloserine inhibition and resistance in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1238–1250. doi: 10.1128/jb.90.5.1238-1250.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo P. M., Bradbeer C. Transport of vitamin B 12 in Escherichia coli. J Bacteriol. 1971 Jun;106(3):745–750. doi: 10.1128/jb.106.3.745-750.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo P. M., Kadner R. J., Bradbeer C. Isolation of vitamin B 12 transport mutants of Escherichia coli. J Bacteriol. 1971 Jun;106(3):751–757. doi: 10.1128/jb.106.3.751-757.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Masi D. R., White J. C., Schnaitman C. A., Bradbeer C. Transport of vitamin B12 in Escherichia coli: common receptor sites for vitamin B12 and the E colicins on the outer membrane of the cell envelope. J Bacteriol. 1973 Aug;115(2):506–513. doi: 10.1128/jb.115.2.506-513.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane M. W. Some effects of nalidixic acid on conjugation in Escherichia coli K-12. J Bacteriol. 1971 Jan;105(1):46–56. doi: 10.1128/jb.105.1.46-56.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper P., Whitney E., Silver S. Genetic locus determining resistance to phage BF23 and colicins E 1 , E 2 and E 3 in Escherichia coli. Genet Res. 1972 Jun;19(3):305–312. doi: 10.1017/s0016672300014555. [DOI] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T., Matsuzawa H., Matsuhashi M., Sugino Y. Cell wall peptidoglycan mutants of Escherichia coli K-12: existence of two clusters of genes, mra and mrb, for cell wall peptidoglycan biosynthesis. J Bacteriol. 1972 Nov;112(2):950–958. doi: 10.1128/jb.112.2.950-958.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orias E., Gartner T. K., Lannan J. E., Betlach M. Close linkage between ochre and missense suppressors in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1125–1133. doi: 10.1128/jb.109.3.1125-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. T., Norrell S. A., Hanna M. L. Uptake of cyanocobalamin by Escherichia coli B: some characteristics and evidence for a binding protein. Arch Biochem Biophys. 1972 Feb;148(2):366–381. doi: 10.1016/0003-9861(72)90154-3. [DOI] [PubMed] [Google Scholar]
- White J. C., DiGirolamo P. M., Fu M. L., Preston Y. A., Bradbeer C. Transport of vitamin B 12 in Escherichia coli. Location and properties of the initial B 12 -binding site. J Biol Chem. 1973 Jun 10;248(11):3978–3986. [PubMed] [Google Scholar]


