Abstract
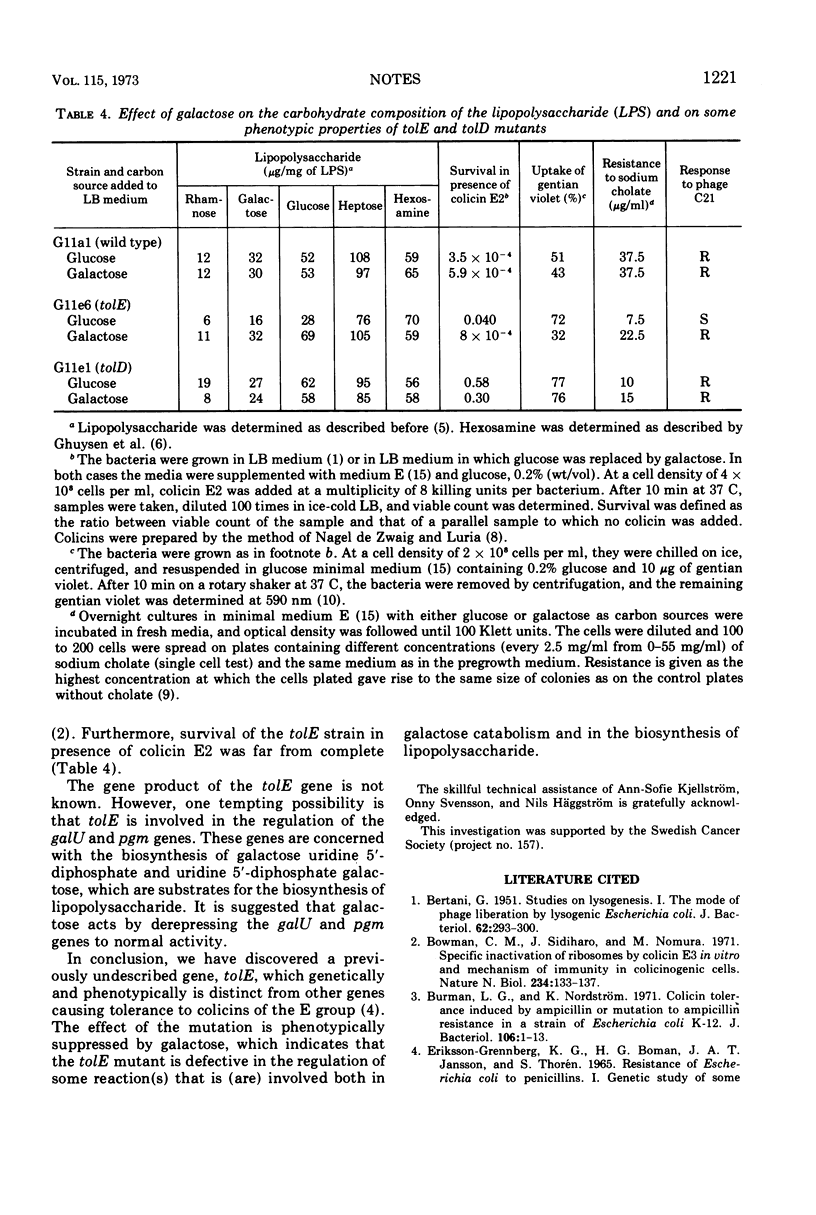
The tolE mutation causes tolerance to colicins E2 and E3 as well as other effects on the phenotype of Escherichia coli K-12. The lipopolysaccharide of the mutant shows a reduction in the content of galactose, glucose, and rhamnose. The phenotype of the mutant, including the composition of the lipopolysaccharide, is suppressed by galactose. The map position is shown by the gene order trp-purB-tolE-tolD-galKETO.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Sidikaro J., Nomura M. Specific inactivation of ribosomes by colicin E3 in vitro and mechanism of immunity in colicinogenic cells. Nat New Biol. 1971 Dec 1;234(48):133–137. doi: 10.1038/newbio234133a0. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K. Colicin tolerance induced by ampicillin or mutation to ampicillin resistance in a strain of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):1–13. doi: 10.1128/jb.106.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. R., Nordström K., Englund P. Resistance of Escherichia coli to penicillins. IX. Genetics and physiology of class II ampicillin-resistant mutants that are galactose negative or sensitive to bacteriophage C21, or both. J Bacteriol. 1971 Dec;108(3):1210–1223. doi: 10.1128/jb.108.3.1210-1223.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Eriksson-Grennberg K. G., Boman H. G. Resistance of Escherichia coli to penicillins. 3. AmpB, a locus affecting episomally and chromosomally mediated resistance to ampicillin and chlorampheincol. Genet Res. 1968 Oct;12(2):157–168. doi: 10.1017/s0016672300011770. [DOI] [PubMed] [Google Scholar]
- Normark S., Westling B. Nature of the penetration barrier in Escherichia coli K-12: effect of macromolecular inhibition of penetrability in strains containing the envA gene. J Bacteriol. 1971 Oct;108(1):45–50. doi: 10.1128/jb.108.1.45-50.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L. E., Meynell E. Specific chromosomal affinity of a resistant factor. J Gen Microbiol. 1968 Jan;50(1):159–172. doi: 10.1099/00221287-50-1-159. [DOI] [PubMed] [Google Scholar]
- Reeve E. C. Genetic analysis of some mutations causing resistance to tetracycline in Escherichia coli K12. Genet Res. 1968 Jun;11(3):303–309. doi: 10.1017/s0016672300011484. [DOI] [PubMed] [Google Scholar]
- SHEDLOVSKY A., BRENNER S. A CHEMICAL BASIS FOR THE HOST-INDUCED MODIFICATION OF T-EVEN BACTERIOPHAGES. Proc Natl Acad Sci U S A. 1963 Aug;50:300–305. doi: 10.1073/pnas.50.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


