Abstract
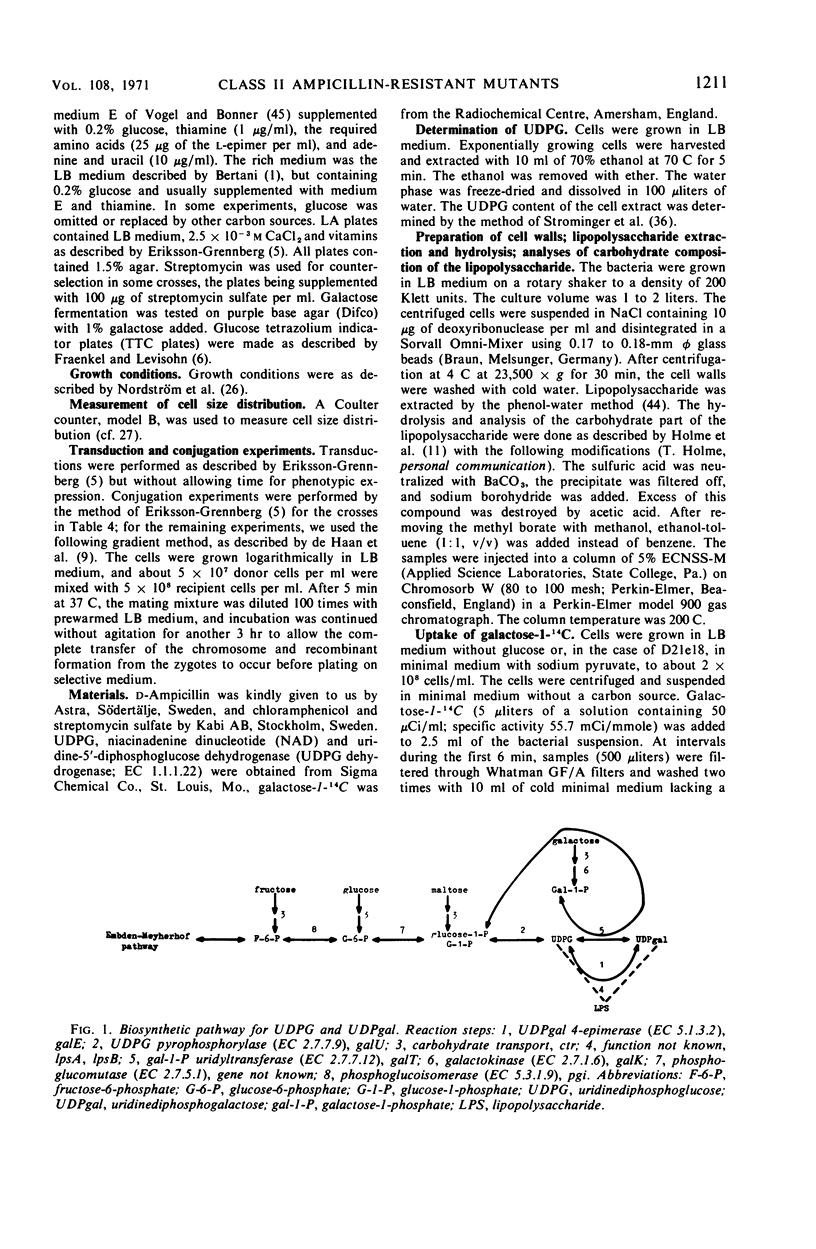
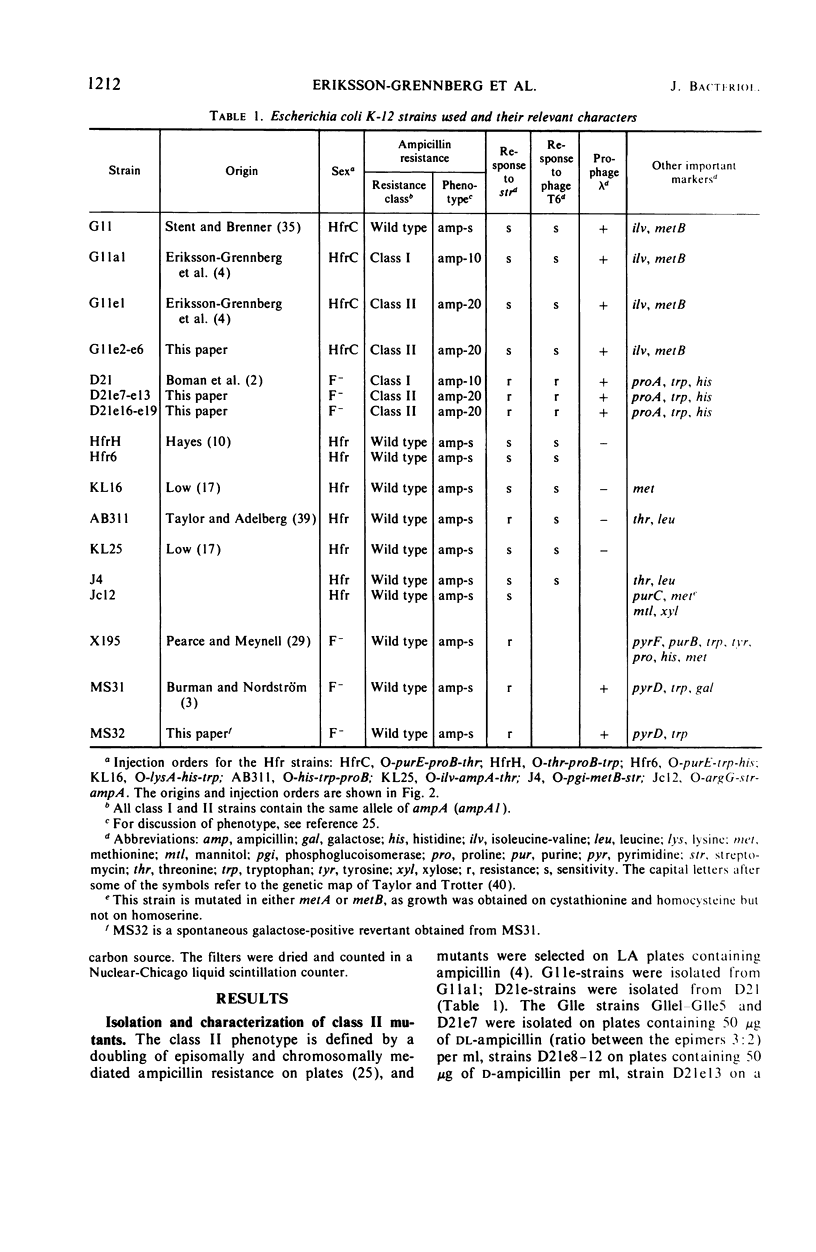
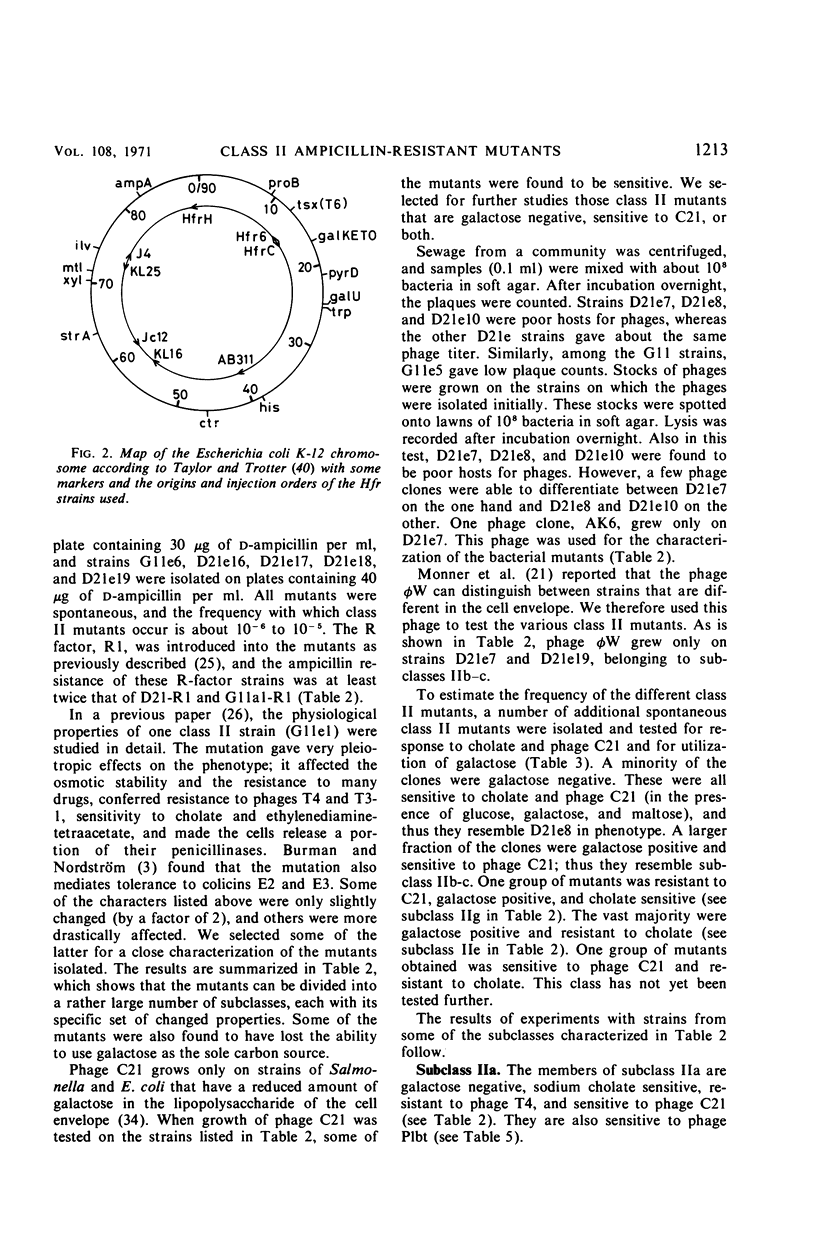
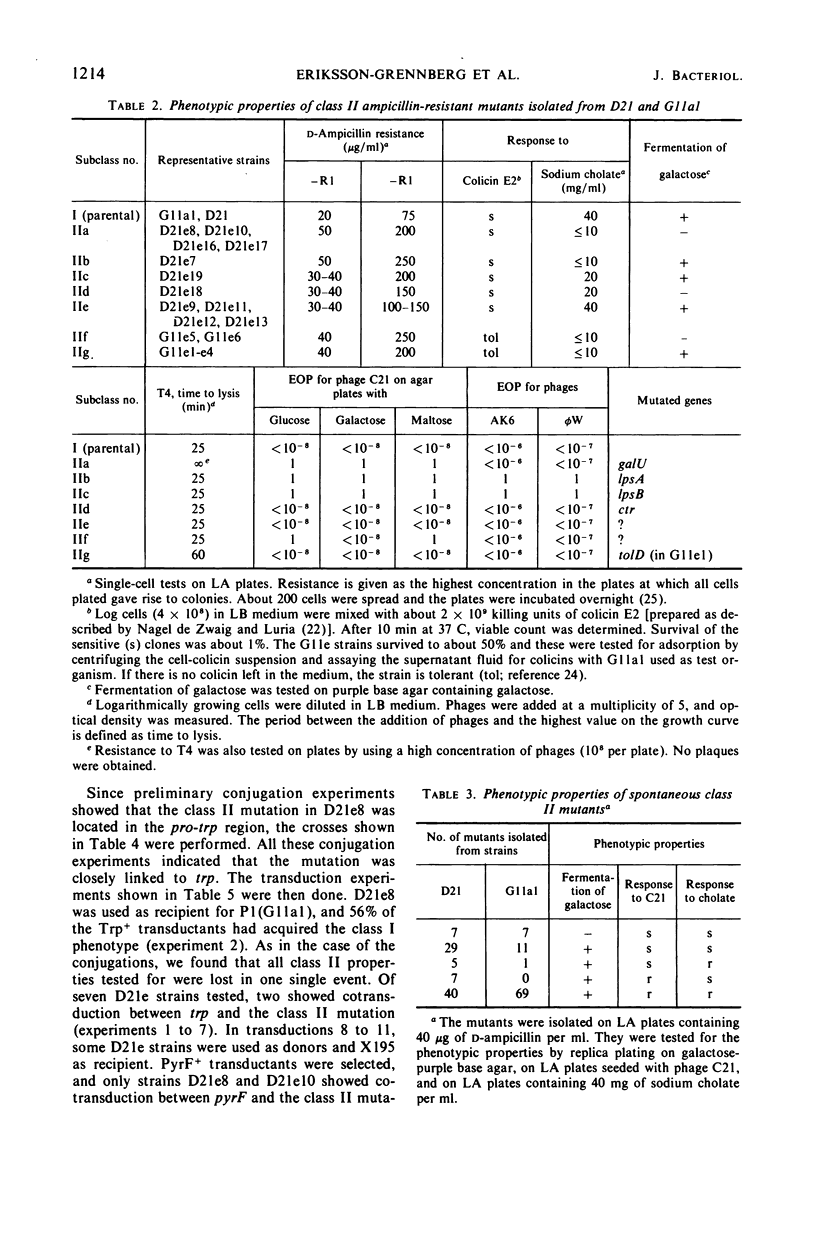
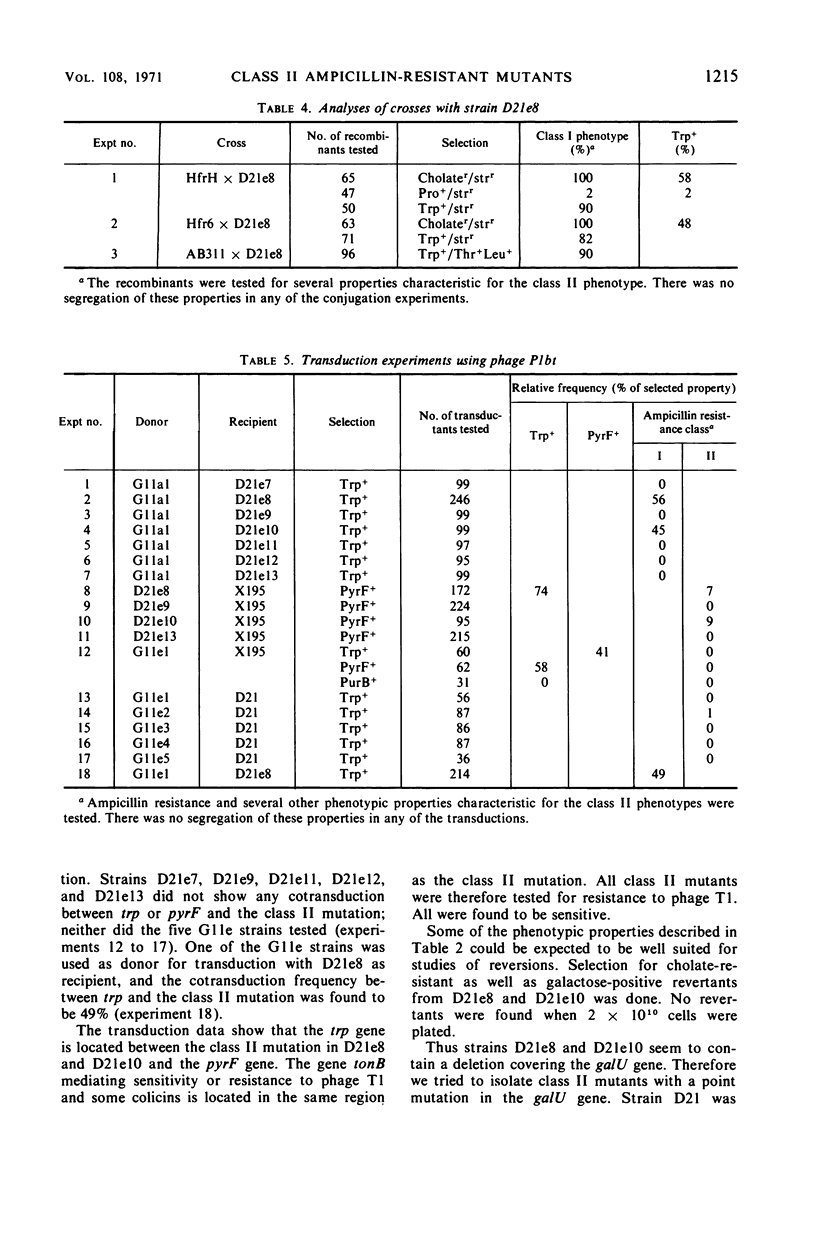
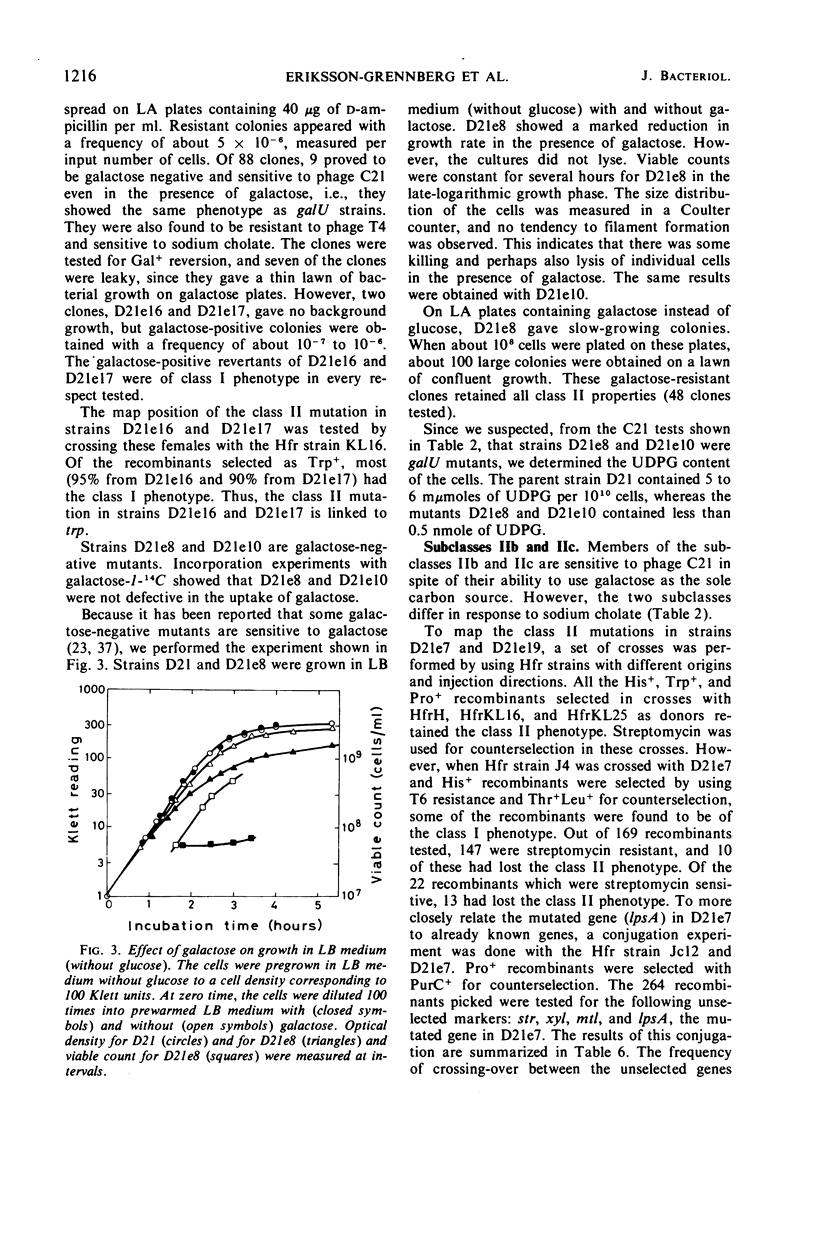
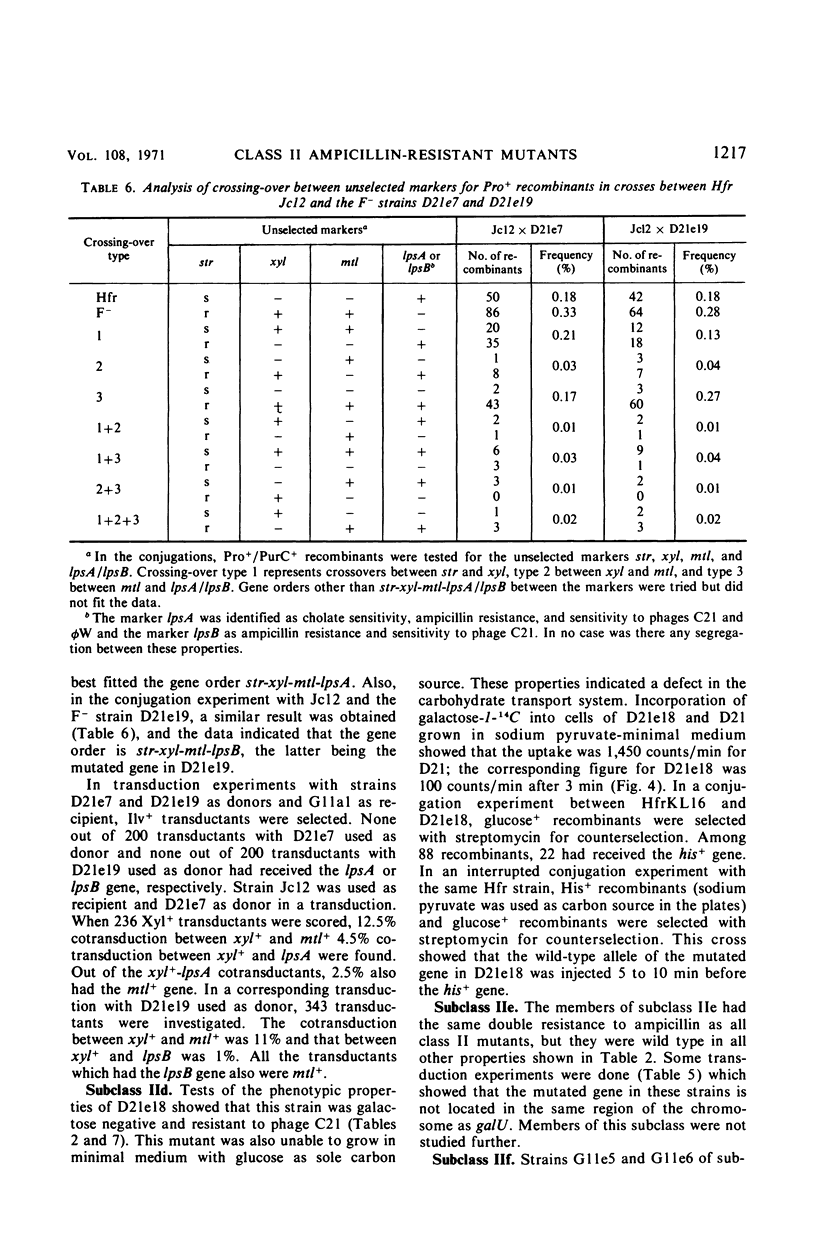
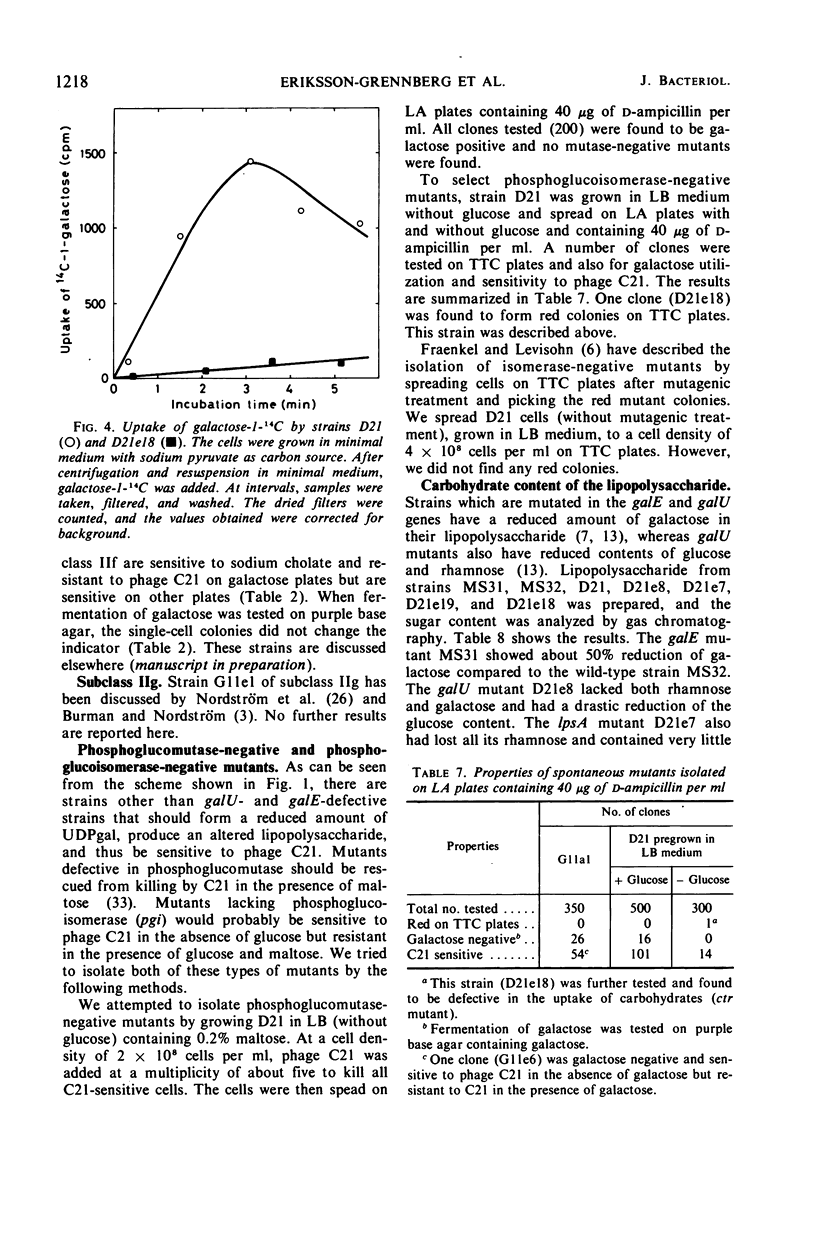
Ampicillin-resistant mutants of class II are determined by a doubling of chromosomally and episomally mediated ampicillin resistance on agar plates. Several mutants were isolated from a female as well as from an Hfr strain. The mutants differed from each other in various properties such as response to colicin E2 and sodium cholate, response to the phages T4 and C21, and fermentation of galactose. By conjugation and transduction experiments, it was shown that mutations in at least four loci gave the class II phenotype. The mutations were found to be in the galU gene, the ctr gene, and two new genes close to mtl denoted lpsA and lpsB. The carbohydrate compositions of the lipopolysaccharides of the mutants were investigated and found to be changed compared to the parent strains. GalU mutants lacked rhamnose and galactose and had 11% glucose compared to the parent strain. The lpsA mutant also lacked rhamnose and had only traces of galactose and 58% glucose, whereas the lpsB mutant contained 14% rhamnose, traces of galactose, and 81% glucose compared to the parent strain.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Eriksson-Grennberg K. G., Normark S., Matsson E. Resistance of Escherichia coli to penicillins. IV. Genetic study of mutants resistant to D,L-ampicillin concentrations o 100 mu-g-ml. Genet Res. 1968 Oct;12(2):169–185. doi: 10.1017/s0016672300011782. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K. Colicin tolerance induced by ampicillin or mutation to ampicillin resistance in a strain of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):1–13. doi: 10.1128/jb.106.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan P. G., Hoekstra W. P., Verhoef C., Felix H. S. Recombination in Escherichia coli. 3. Mapping by the gradient of transmission. Mutat Res. 1969 Nov-Dec;8(3):505–512. doi: 10.1016/0027-5107(69)90067-0. [DOI] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Boman H. G., Jansson J. A., Thorén S. Resistance of Escherichia coli to Penicillins I. Genetic Study of Some Ampicillin-Resistant Mutants. J Bacteriol. 1965 Jul;90(1):54–62. doi: 10.1128/jb.90.1.54-62.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. II. An improved mapping of the ampA gene. Genet Res. 1968 Oct;12(2):147–156. doi: 10.1017/s0016672300011769. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., JOKURA K., KURAHASHI K. MUTATIONS IN ESCHERICHIA COLI THAT AFFECT URIDINE DIPHOSPHATE GLUCOSE PYROPHOSPHORYLASE ACTIVITY AND GALACTOSE FERMENTATION. Biochim Biophys Acta. 1963 Sep 10;74:608–620. doi: 10.1016/0006-3002(63)91412-4. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G., Levisohn S. R. Glucose and gluconate metabolism in an Escherichia coli mutant lacking phosphoglucose isomerase. J Bacteriol. 1967 May;93(5):1571–1578. doi: 10.1128/jb.93.5.1571-1578.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratia J. P. Studies on defective lysogeny due to chromosomal deletion in Escherichia coli. I. Single lysogens. Biken J. 1966 Jun;9(2):77–87. [PubMed] [Google Scholar]
- Horecker B. L. The biosynthesis of bacterial polysaccharides. Annu Rev Microbiol. 1966;20:253–290. doi: 10.1146/annurev.mi.20.100166.001345. [DOI] [PubMed] [Google Scholar]
- Kalckar H. M., Laursen P., Rapin A. M. Inactivation of phage c21 by various preparations from lipopolysaccharide of e. Coli k-12. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1852–1858. doi: 10.1073/pnas.56.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Linström E. B., Boman H. G., Steele B. B. Resistance of Escherichia coli to penicillins. VI. Purification and characterization of the chromosomally mediated penicillinase present in ampA-containing strains. J Bacteriol. 1970 Jan;101(1):218–231. doi: 10.1128/jb.101.1.218-231.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Monner D. A., Boman H. G. Female strains of Escherichia coli K12 as selective hosts for the isolation of female specific mutants of phage omega II. Biochem Biophys Res Commun. 1970;39(6):1017–1020. doi: 10.1016/0006-291x(70)90659-5. [DOI] [PubMed] [Google Scholar]
- Monner D. A., Jonsson S., Boman H. G. Ampicillin-resistant mutants of Escherichia coli K-12 with lipopolysaccharide alterations affecting mating ability and susceptibility to sex-specific bacteriophages. J Bacteriol. 1971 Aug;107(2):420–432. doi: 10.1128/jb.107.2.420-432.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIKAIDO H. Galactose-sensitive mutants of Salmonella. I. Metabolism of galactose. Biochim Biophys Acta. 1961 Apr 15;48:460–469. doi: 10.1016/0006-3002(61)90044-0. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Burman L. G., Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. 8. Physiology of a class II ampicillin-resistant mutant. J Bacteriol. 1970 Mar;101(3):659–668. doi: 10.1128/jb.101.3.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Eriksson-Grennberg K. G., Boman H. G. Resistance of Escherichia coli to penicillins. 3. AmpB, a locus affecting episomally and chromosomally mediated resistance to ampicillin and chlorampheincol. Genet Res. 1968 Oct;12(2):157–168. doi: 10.1017/s0016672300011770. [DOI] [PubMed] [Google Scholar]
- Normark S. Genetics of a chain-forming mutant of Escherichia coli. Transduction and dominance of the envA gene mediating increased penetration to some antibacterial agents. Genet Res. 1970 Aug;16(1):63–78. doi: 10.1017/s0016672300002287. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., ZELEZNICK L. D., HORECKER B. L. LIPOPOLYSACCHARIDE OF THE GRAM-NEGATIVE CELL WALL. Science. 1964 Aug 21;145(3634):783–789. doi: 10.1126/science.145.3634.783. [DOI] [PubMed] [Google Scholar]
- Pearce L. E., Meynell E. Specific chromosomal affinity of a resistant factor. J Gen Microbiol. 1968 Jan;50(1):159–172. doi: 10.1099/00221287-50-1-159. [DOI] [PubMed] [Google Scholar]
- Rapin A. M., Mayer H. Complex polysaccharides in different strains of Escherichia coli K-12. Ann N Y Acad Sci. 1966 Jun 30;133(2):425–437. doi: 10.1111/j.1749-6632.1966.tb52381.x. [DOI] [PubMed] [Google Scholar]
- SHEDLOVSKY A., BRENNER S. A CHEMICAL BASIS FOR THE HOST-INDUCED MODIFICATION OF T-EVEN BACTERIOPHAGES. Proc Natl Acad Sci U S A. 1963 Aug;50:300–305. doi: 10.1073/pnas.50.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNDARARAJAN T. A., RAPIN A. M., KALCKAR H. M. Biochemical observations on E. coli mutants defective in uridine diphosphoglucose. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2187–2193. doi: 10.1073/pnas.48.12.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Current linkage map of Salmonella typhimurium. Bacteriol Rev. 1970 Jun;34(2):176–193. doi: 10.1128/br.34.2.176-193.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K. Immunochemistry of R lipopolysaccharides of Escherichia coli. Studies on R mutants with an incomplete core, derived from E. coli O8:K27. Eur J Biochem. 1970 Oct;16(2):382–392. doi: 10.1111/j.1432-1033.1970.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Chromosomal location of the gene determining uridine diphoglucose formation in Escherichia coli K-12. J Bacteriol. 1966 Aug;92(2):518–520. doi: 10.1128/jb.92.2.518-520.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A L, Adelberg E A. Linkage Analysis with Very High Frequency Males of Escherichia Coli. Genetics. 1960 Sep;45(9):1233–1243. doi: 10.1093/genetics/45.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wang R. J., Morse H. G., Morse M. L. Carbohydrate accumulation and metabolism in Escherichia coli: the close linkage and chromosomal location of ctr mutations. J Bacteriol. 1969 May;98(2):605–610. doi: 10.1128/jb.98.2.605-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. J., Morse M. L. Carbohydrate accumulation and metabolism in Escherichia coli. I. Description of pleiotropic mutants. J Mol Biol. 1968 Feb 28;32(1):59–66. doi: 10.1016/0022-2836(68)90145-9. [DOI] [PubMed] [Google Scholar]


