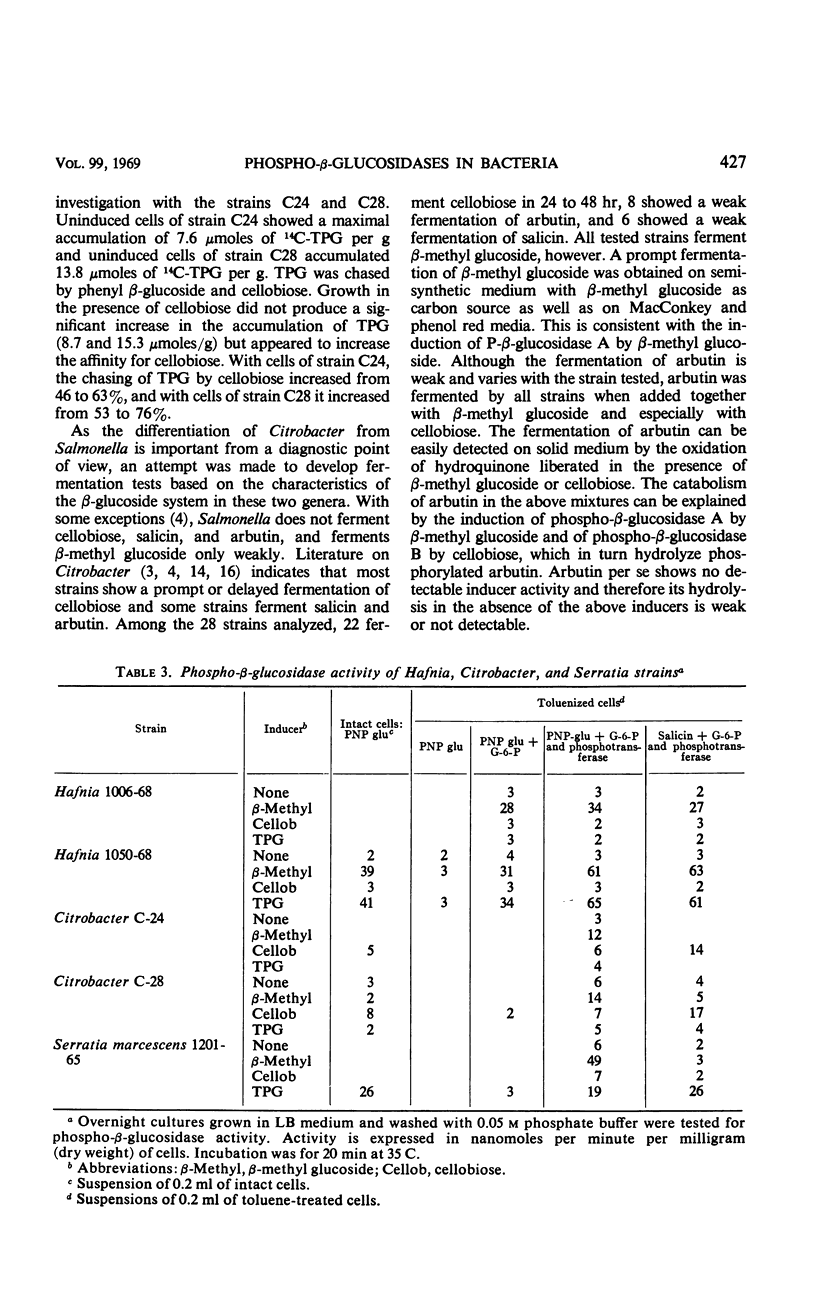
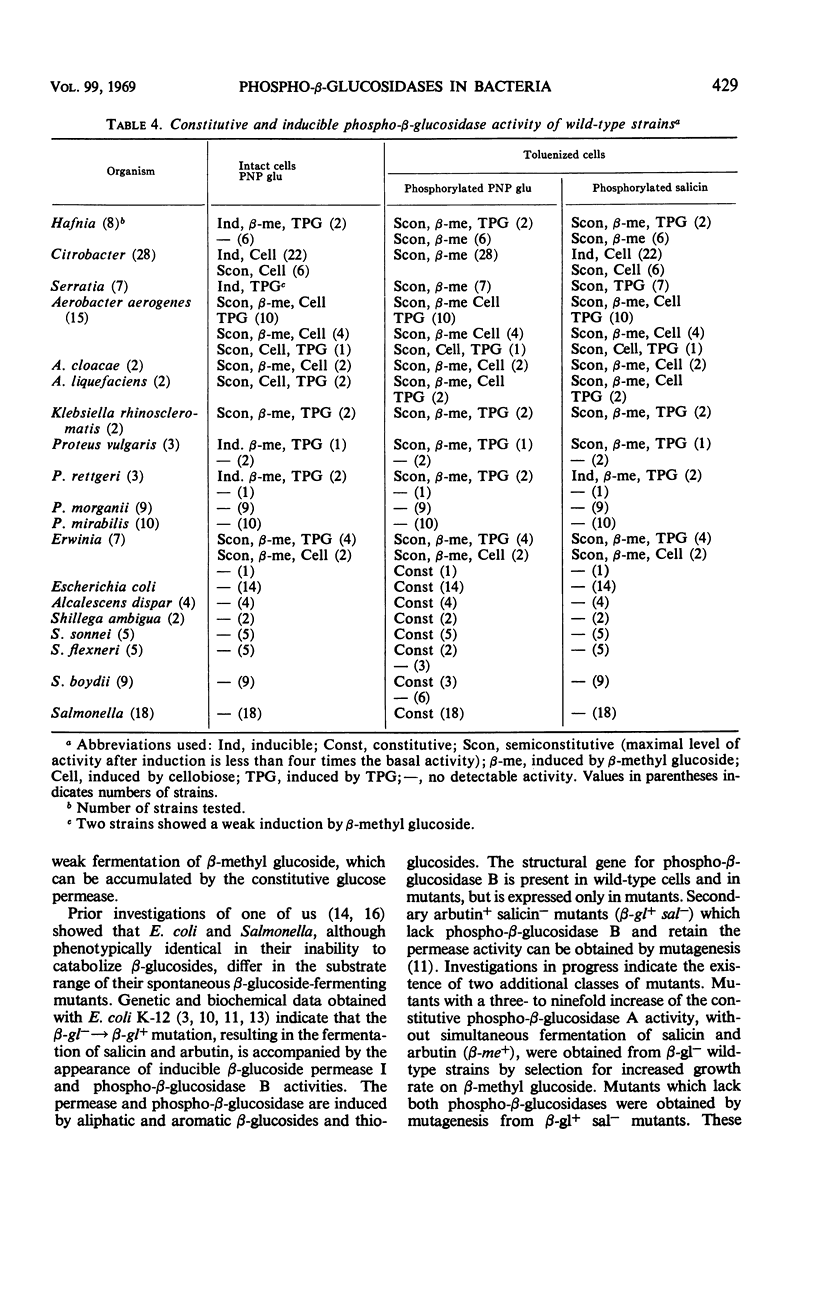
Abstract
In the Enterobacteriaceae, β-glucosides are catabolized by a complex system formed of three permeases, with partly overlapping substrate specificities, and two hydrolytic enzymes, phospho-β-glucosidase A and B, which hydrolyze only phosphorylated β-glucosides. Some Enterobacteriaceae such as Klebsiella-Aerobacter (Enterobacter) possess the complete system; others possess only parts of it or may have a cryptic phospho-β-glucosidase activity without permease activity. A screening test applied to strains belonging to several genera of Enterobacteriaceae showed that strains of Citrobacter, Hafnia, and Serratia exhibit a degree of similarity in phospho-β-glucosidase activity and inducibility which could be useful in their taxonomic characterization; others, such as Aerobacter aerogenes, Erwinia, and Proteus vulgaris, are more heterologous. Owing to the presence of inducible phospho-β-glucosidases A and B in Citrobacter, the fermentation of β-methyl glucoside and the fermentation of arbutin in mixture with cellobiose could be of diagnostic value in the differentiation of Citrobacter from Salmonella. Wild-type strains of Escherichia coli, Shigella, and Salmonella are phenotypically similar in their inability to catabolize β-glucosides, the presence of constitutive P-β-glucosidase A, and the lack of β-glucoside permeases I and II. Their β-glucoside-fermenting mutants show, however, a phospho-β-glucosidase and β-glucoside permease activity which is characteristic for mutants from each genus. The differences in the phenotype of the mutants reflect probable differences in the presence of cryptic genes in the wild-type strains and could be of evolutionary significance.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Wilson G. The role of a phosphoenolpyruvate-dependent kinase system in beta-glucoside catabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Mar;59(3):988–995. doi: 10.1073/pnas.59.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Morgenroth A., Duguid J. P. Demonstration of different mutational sites controlling rhamnose fermentation in FIRN and non-FIRN rha-strains of Salmonella typhimurium: an essay in bacterial archaeology. Genet Res. 1968 Apr;11(2):151–169. doi: 10.1017/s0016672300011320. [DOI] [PubMed] [Google Scholar]
- SCHAFLER S., MINTZER L. Acquisition of lactose-fermenting properties by salmonellae. I. Interrelationship between the fermentation of cellobiose and lactose. J Bacteriol. 1959 Aug;78:159–163. doi: 10.1128/jb.78.2.159-163.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFLER S., SCHAFLER C. Etude comparative sur des souches d'Escherichia freundii et des souches de Salmonella. Ann Inst Pasteur (Paris) 1959 Jun;96(6):790–794. [PubMed] [Google Scholar]
- Schaefler S. Inducible system for the utilization of beta-glucosides in Escherichia coli. I. Active transport and utilization of beta-glucosides. J Bacteriol. 1967 Jan;93(1):254–263. doi: 10.1128/jb.93.1.254-263.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Maas W. K. Inducible system for the utilization of beta-glucosides in Escherichia coli. II. Description of mutant types and genetic analysis. J Bacteriol. 1967 Jan;93(1):264–272. doi: 10.1128/jb.93.1.264-272.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Malamy A., Green I. Phospho-beta-glucosidases and beta-glucoside permeases in Streptococcus, Bacillus, and Staphylococcus. J Bacteriol. 1969 Aug;99(2):434–440. doi: 10.1128/jb.99.2.434-440.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Schenkein I. Beta-glucoside permeases and phospho beta-glucosidases in Aerobacter aerogenes: relationship with cryptic phospho beta-glucosidases in Enterobacteriaceae. Proc Natl Acad Sci U S A. 1968 Jan;59(1):285–292. doi: 10.1073/pnas.59.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamenhof S., Eichhorn H. H. Study of microbial evolution through loss of biosynthetic functions: establishment of "defective" mutants. Nature. 1967 Nov 4;216(5114):456–458. doi: 10.1038/216456a0. [DOI] [PubMed] [Google Scholar]


