Abstract
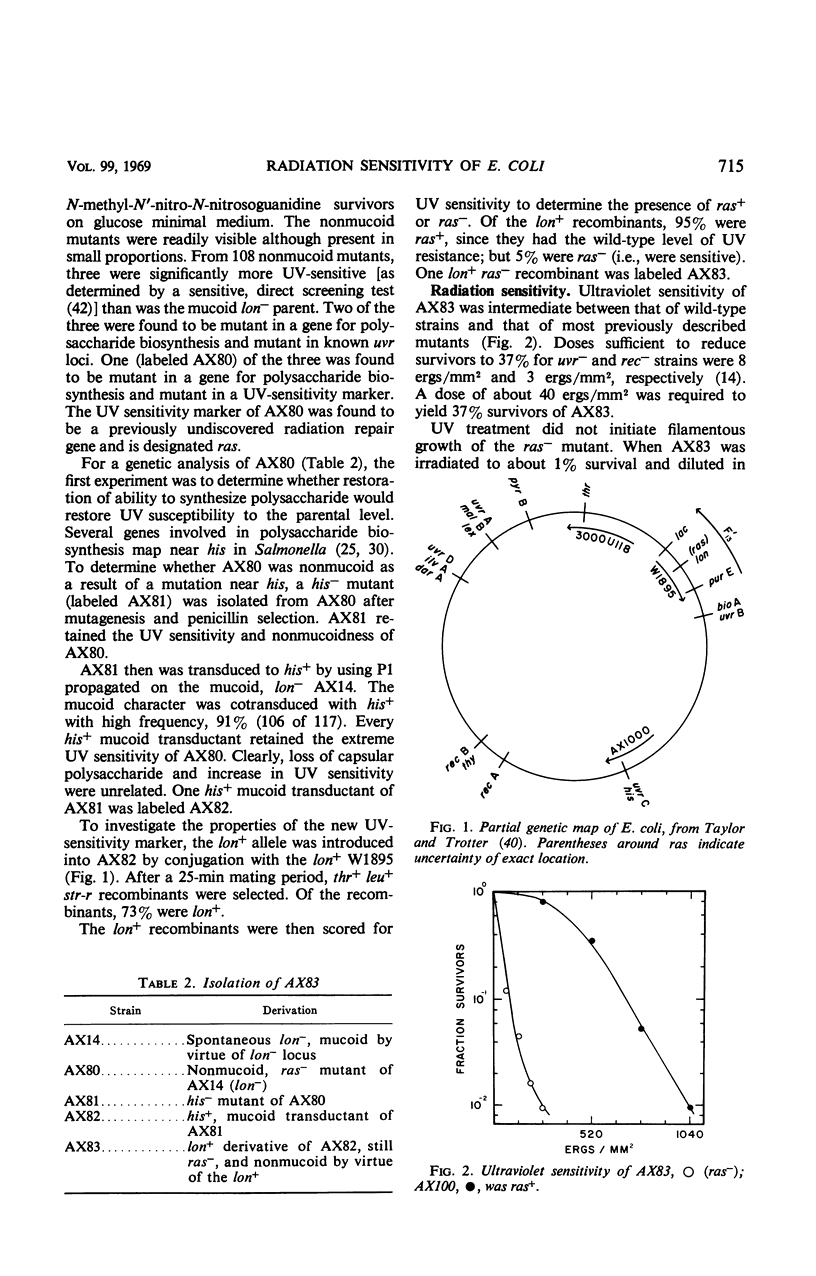
There are several classes of Escherichia coli mutants defective in radiation repair. These include strains defective in pyrimidine dimer excision, in photoreactivation, in recombination, in repair of X-ray damage, and ultraviolet (UV)-conditional mutants which do not divide after UV. Another mutant (ras−) has been isolated. The ras− has increased UV sensitivity, but only slightly increased X-ray sensitivity (1.5-fold increase). Ability to effect genetic recombination, to reactivate irradiated bacteriophage T1, and to be photoreactivated is normal. UV-induced mutation frequency is greatly increased in the mutant. The ras− apparently lacks the ability to repair some UV damage in the bacterial cell but can repair UV damage to bacteriophage DNA. The ras locus is located between lac and purE on the chromosome map.
Full text
PDF
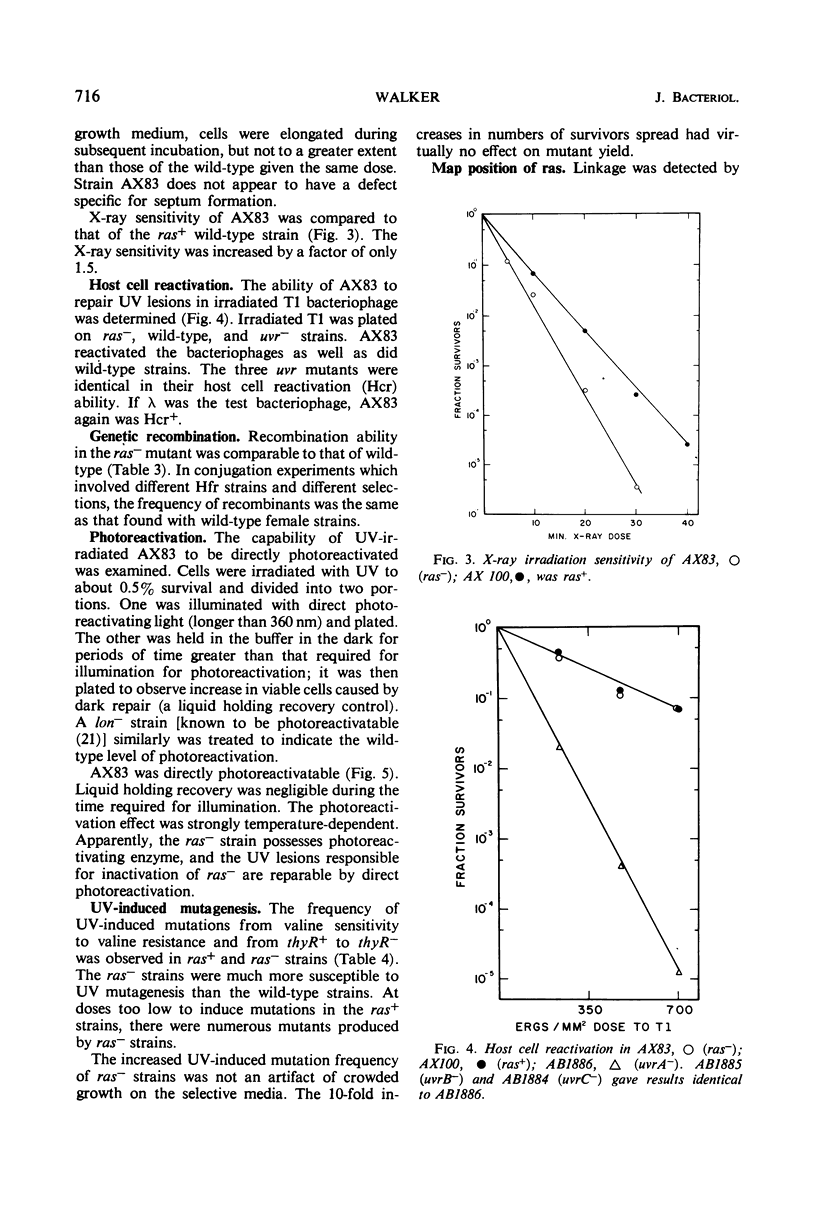
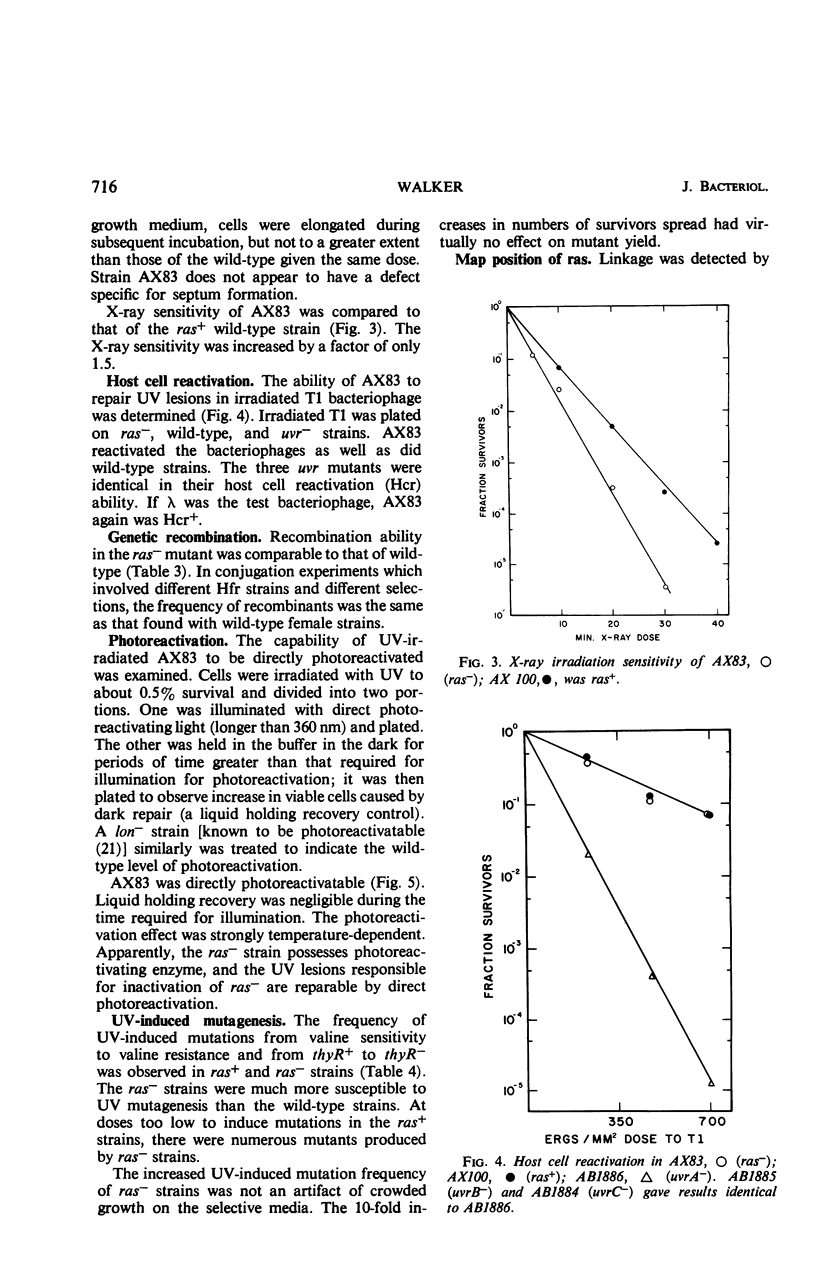
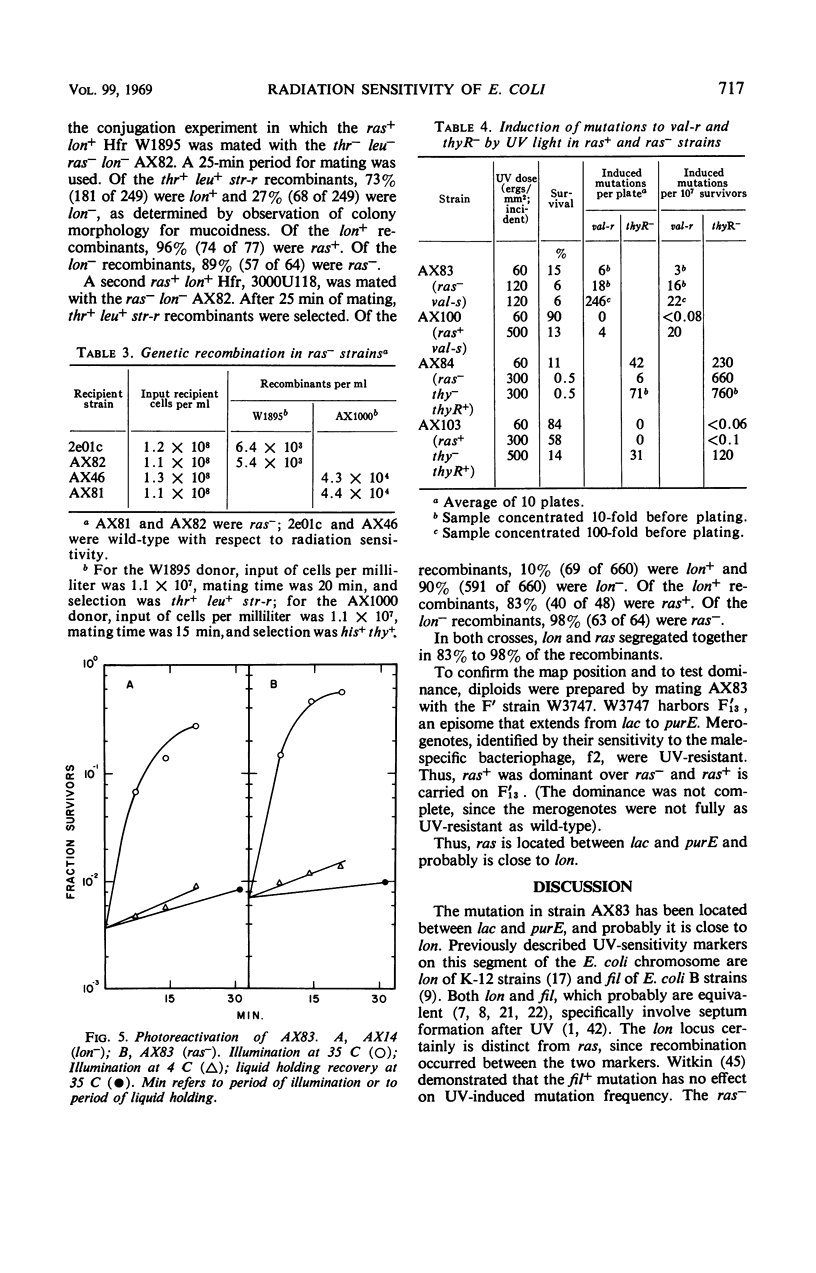


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Hardigree A. A. Growth and Division of Filamentous Forms of Escherichia coli. J Bacteriol. 1965 Jul;90(1):223–226. doi: 10.1128/jb.90.1.223-226.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Genetic studies of strain Bs8 of Escherichia coli. Genet Res. 1968 Aug;12(1):55–63. doi: 10.1017/s0016672300011617. [DOI] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Loci of radiation sensitivity in Bs strains of Escherichia coli. Genet Res. 1968 Apr;11(2):183–191. doi: 10.1017/s0016672300011356. [DOI] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Ultraviolet sensitivity gene of Escherichia coli B. J Bacteriol. 1968 May;95(5):1555–1559. doi: 10.1128/jb.95.5.1555-1559.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG J. A LOCUS FOR RADIATION RESISTANCE IN ESCHERICHIA COLI. Genetics. 1964 May;49:771–778. doi: 10.1093/genetics/49.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. Radiation sensitivity in Escherichia coli: some properties of the radiation-sensitive Hfr K12 mutant, PAM 401. Mutat Res. 1965 Aug;2(4):304–311. doi: 10.1016/0027-5107(65)90064-3. [DOI] [PubMed] [Google Scholar]
- HILL R. F., SIMSON E. A study of radiosensitive and radioresistant mutants of Escherichia coli strain B. J Gen Microbiol. 1961 Jan;24:1–14. doi: 10.1099/00221287-24-1-1. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., BOYCE R. P., SIMSON E., THERIOT L. A genetic locus in E. coli K12 that controls the reactivation of UV-photoproducts associated with thymine in DNA. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2109–2115. doi: 10.1073/pnas.48.12.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. F. Ultraviolet-induced lethality and reversion to prototrophy in Escherichia coli strains with normal and reduced dark repair ability. Photochem Photobiol. 1965 Jun;4(3):563–568. doi: 10.1111/j.1751-1097.1965.tb09774.x. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. I. Molecular origin of the fragments. J Mol Biol. 1965 Nov;14(1):85–109. doi: 10.1016/s0022-2836(65)80232-7. [DOI] [PubMed] [Google Scholar]
- JAGGER J., STAFFORD R. S. EVIDENCE FOR TWO MECHANISMS OF PHOTOREACTIVATION IN ESCHERICHIA COLI B. Biophys J. 1965 Jan;5:75–88. doi: 10.1016/s0006-3495(65)86703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor G. J., Deering R. A. Effect of nalidixic acid and hydroxyurea on division ability of Escherichia coli fil+ and lon- strains. J Bacteriol. 1968 Feb;95(2):520–530. doi: 10.1128/jb.95.2.520-530.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor G. J., Deering R. A. Recovery of division ability in ultraviolet-irradiated Escherichia coli induced by photoreactivation, photoprotection, and liquid holding treatment. J Bacteriol. 1967 Dec;94(6):1946–1950. doi: 10.1128/jb.94.6.1946-1950.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEB T., ZINDER N. D. A bacteriophage containing RNA. Proc Natl Acad Sci U S A. 1961 Mar 15;47:282–289. doi: 10.1073/pnas.47.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- MANTEN A., ROWLEY D. Genetic analysis of valine inhibition in the K12 strain of Bacterium coli. J Gen Microbiol. 1953 Oct;9(2):226–233. doi: 10.1099/00221287-9-2-226. [DOI] [PubMed] [Google Scholar]
- MOROWITZ H. J. Absorption effects in volume irradiation of microorganisms. Science. 1950 Mar 3;111(2879):229–229. doi: 10.1126/science.111.2879.229-a. [DOI] [PubMed] [Google Scholar]
- Mattern I. E., Zwenk H., Rörsch A. The genetic constitution of the radiation-sensitive mutant Escherichia coli Bs-1. Mutat Res. 1966 Oct;3(5):374–380. doi: 10.1016/0027-5107(66)90047-9. [DOI] [PubMed] [Google Scholar]
- Miura A., Tomizawa J. I. Studies on radiation-sensitive mutants of E. coli. 3. Participation of the rec system in induction of mutation by ultraviolet irradiation. Mol Gen Genet. 1968;103(1):1–10. doi: 10.1007/BF00271151. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H. Genetic determination of the O antigens of Salmonella groups B (4,5,12) and C1(6,7). J Bacteriol. 1966 Mar;91(3):1115–1125. doi: 10.1128/jb.91.3.1115-1125.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nikaido K., Mäkelä P. H. Genetic determination of enzymes synthesizing O-specific sugars of Salmonella lipopolysaccharides. J Bacteriol. 1966 Mar;91(3):1126–1135. doi: 10.1128/jb.91.3.1126-1135.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- Okada T. Mutational Site of the Gene Controlling Quantitative Thymine Requirement in ESCHERICHIA COLI K-12. Genetics. 1966 Dec;54(6):1329–1336. doi: 10.1093/genetics/54.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RORSCH A., EDELMAN A., COHEN J. A. The gene-controlled radiation sensitivity in Escherichia coli. Biochim Biophys Acta. 1963 Feb 26;68:263–270. doi: 10.1016/0006-3002(63)90141-0. [DOI] [PubMed] [Google Scholar]
- RORSCH A., EDELMAN A., van der KAMP, COHEN J. A. Phenotypic and genotypic characterization of radiation sensitivity in Escherichia coli B. Biochim Biophys Acta. 1962 Aug 20;61:278–289. doi: 10.1016/0926-6550(62)90090-7. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- SADLER J. R., NOVICK A. THE PROPERTIES OF REPRESSOR AND THE KINETICS OF ITS ACTION. J Mol Biol. 1965 Jun;12:305–327. doi: 10.1016/s0022-2836(65)80255-8. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Carrier W. L., Setlow R. B. Photoreactivation in vivo of pyrimidine dimers in paramecium DNA. Science. 1967 Dec 29;158(3809):1699–1700. doi: 10.1126/science.158.3809.1699. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WULFF D. L., RUPERT C. S. Disappearance of thymine photodimer in ultraviolet irradiated DNA upon treatment with a photoreactivating enzyme from baker's yeast. Biochem Biophys Res Commun. 1962 Apr 20;7:237–240. doi: 10.1016/0006-291x(62)90181-x. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Conditional mutations involving septum formation in Escherichia coli. J Bacteriol. 1967 Jan;93(1):107–114. doi: 10.1128/jb.93.1.107-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Evidence for a relationship between deoxyribonucleic acid metabolism and septum formation in Escherichia coli. J Bacteriol. 1968 Jan;95(1):123–131. doi: 10.1128/jb.95.1.123-131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Radiation-induced mutations and their repair. Science. 1966 Jun 3;152(3727):1345–1353. doi: 10.1126/science.152.3727.1345. [DOI] [PubMed] [Google Scholar]


