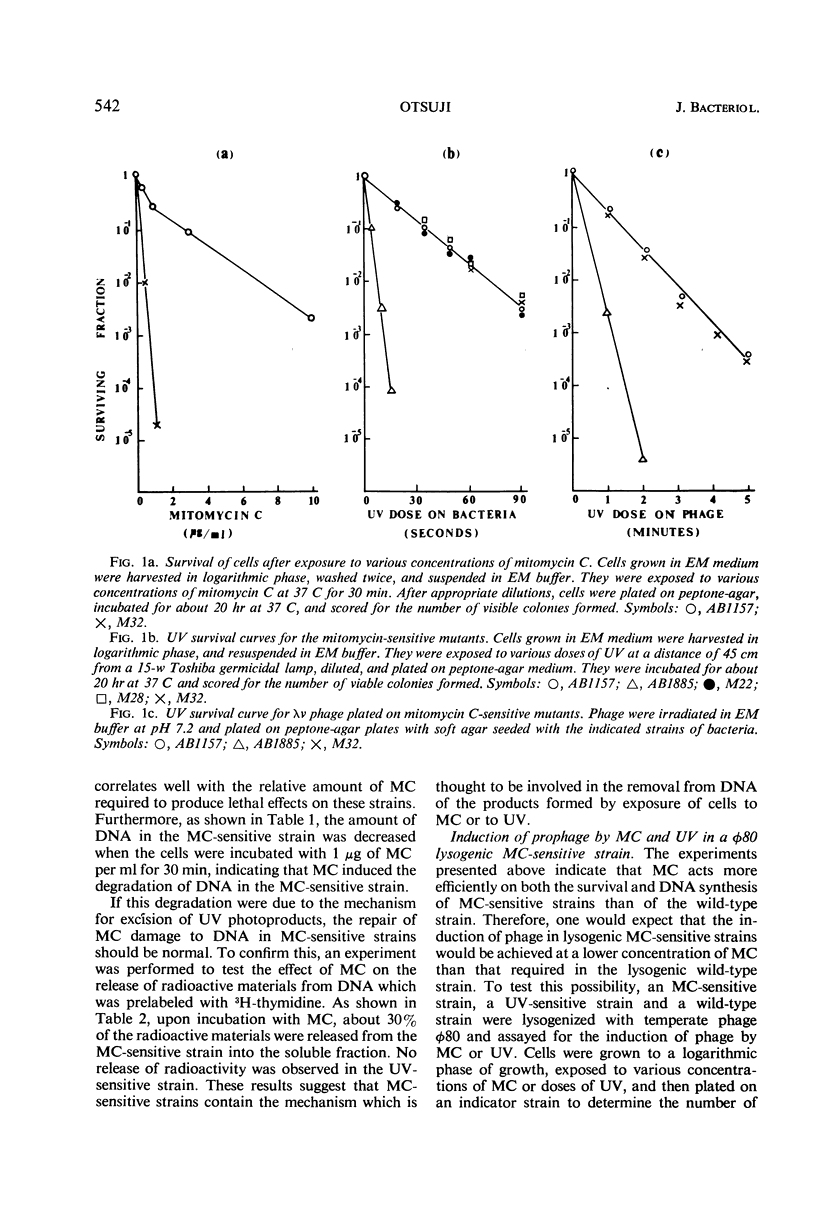
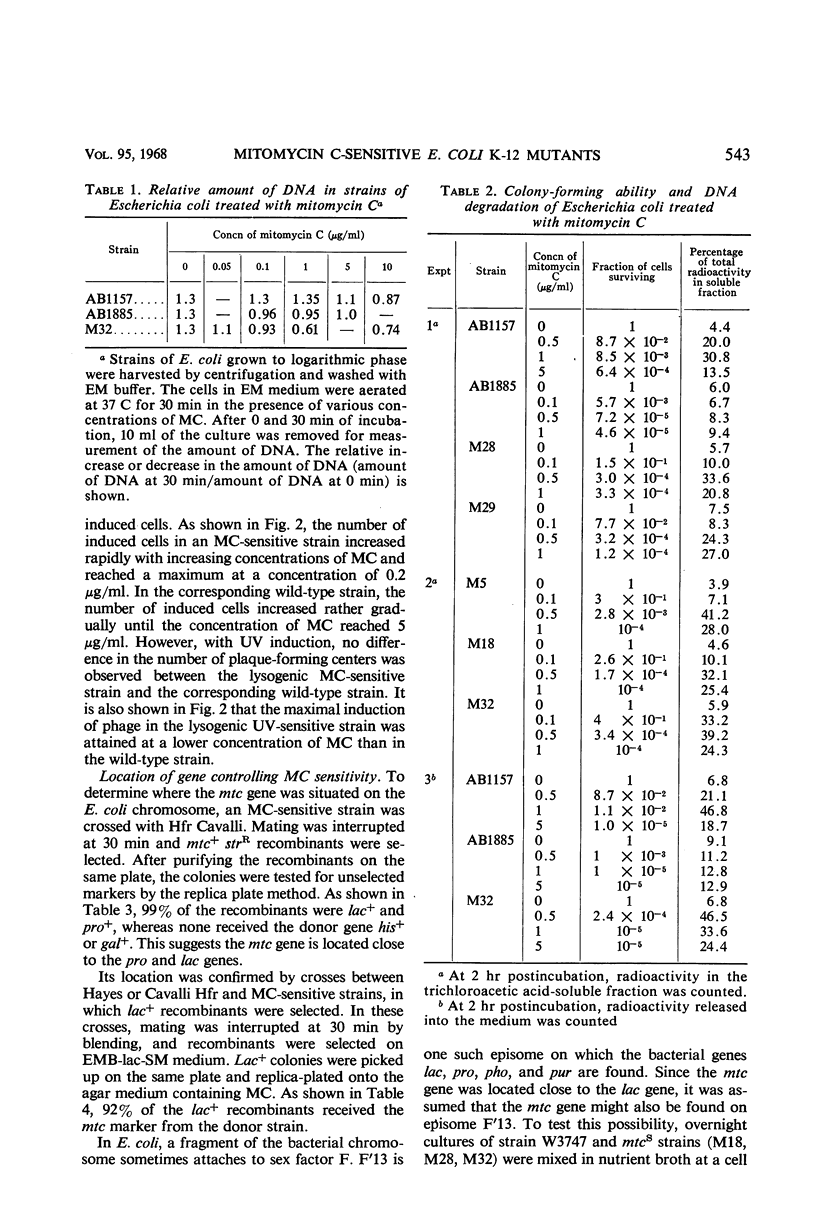
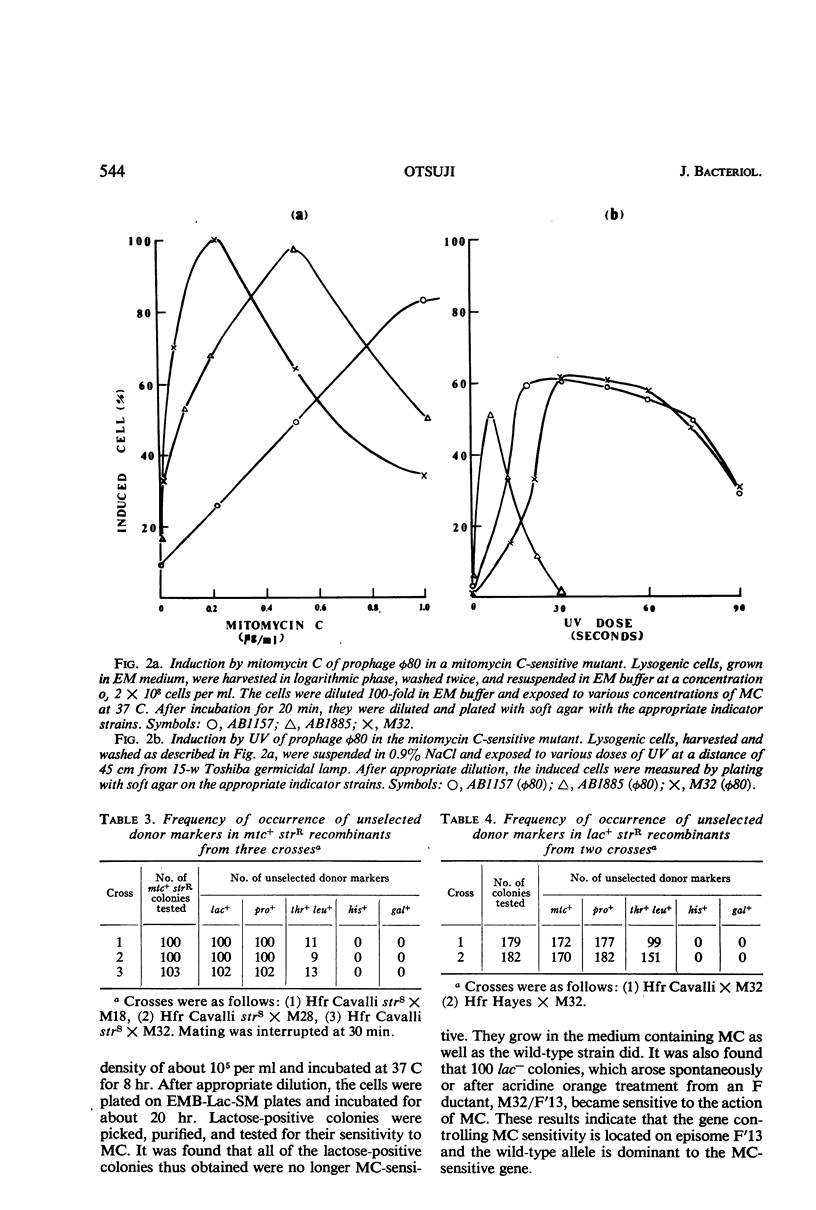
Abstract
Strains hypersensitive to mitomycin C (MC) were isolated from Escherichia coli K-12 after treatment with nitrosoguanidine. Of 43 MC-sensitive strains tested for their ultraviolet light (UV) sensitivity and for their ability to reactivate UV-inactivated λ phage, 38 were found to be insensitive to UV irradiation and to be able to reactivate UV-irradiated bacteriophage λ. Some properties of the MC-sensitive, uvr+ mutants were analyzed. Synthesis of deoxyribonucleic acid (DNA) in MC-sensitive, uvr+ mutants was inhibited at a lower concentration of MC than in the wild-type strain. Mutant cells, labeled with 3H-thymidine and then exposed to MC, released radioactivity as low molecular weight compounds. The amount of radioactivity released was the same as that from the wild-type strain. MC-sensitive, uvr+ mutants, as well as the corresponding wild-type strain, were equally susceptible to induction of prophage φ80 by UV irradiation. However, MC induction of prophage was achieved in MC-sensitive, uvr+ mutants at a lower concentration of the antibiotic than in the wild-type strain. Genetic experiments indicated that a gene controlling MC sensitivity is located close to that determining lactose fermentation of E. coli. It is situated on episome F′13, and the wild type is dominant to the MC-sensitive allele.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. GENETIC CONTROL OF DNA BREAKDOWN AND REPAIR IN E. COLI K-12 TREATED WITH MITOMYCIN C OR ULTRAVIOLET LIGHT. Z Vererbungsl. 1964 Dec 30;95:345–350. doi: 10.1007/BF01268667. [DOI] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG J., MANDELL J. D., WOODY P. L. Resistance and cross-resistance of Escherichia coli mutants to antitumour agent mitomycin C. J Gen Microbiol. 1961 Nov;26:509–520. doi: 10.1099/00221287-26-3-509. [DOI] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. MITOMYCINS AND PORFIROMYCIN: CHEMICAL MECHANISM OF ACTIVATION AND CROSS-LINKING OF DNA. Science. 1964 Jul 3;145(3627):55–58. doi: 10.1126/science.145.3627.55. [DOI] [PubMed] [Google Scholar]
- OTSUJI N., OKUBO S. Reactivation of ultraviolet- and nitrous acid-inactivated phages by host cells. Virology. 1960 Dec;12:607–609. doi: 10.1016/0042-6822(60)90185-9. [DOI] [PubMed] [Google Scholar]
- OTSUJI N., SEKIGUCHI M., IIJIMA T., TAKAGI Y. Induction of phage formation in the lysogenic Escherichia coli K-12 by mitomycin C. Nature. 1959 Oct 3;184(Suppl 14):1079–1080. doi: 10.1038/1841079b0. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Impaired transformability of Bacillus subtilis mutant sensitive to mitomycin C and ultraviolet radiation. J Mol Biol. 1966 Feb;15(2):440–454. doi: 10.1016/s0022-2836(66)80120-1. [DOI] [PubMed] [Google Scholar]
- REICH E., SHATKIN A. J., TATUM E. L. Bacteriocidal action of mitomycin C. Biochim Biophys Acta. 1961 Oct 14;53:132–149. doi: 10.1016/0006-3002(61)90800-9. [DOI] [PubMed] [Google Scholar]
- SEKIGUCHI M., TAKAGI Y. Effect of mitomycin C on the synthesis of bacterial and viral deoxyribonucleic acid. Biochim Biophys Acta. 1960 Jul 15;41:434–443. doi: 10.1016/0006-3002(60)90040-8. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBA S., TERAWAKI A., TAGUCHI T., KAWAMATA J. Selective inhibition of formation of deoxyribonucleic acid in Escherichia coli by mitomycin C. Nature. 1959 Apr 11;183(4667):1056–1057. doi: 10.1038/1831056a0. [DOI] [PubMed] [Google Scholar]


