Abstract
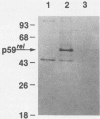
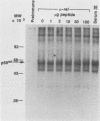
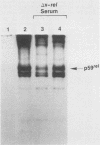
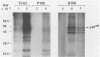
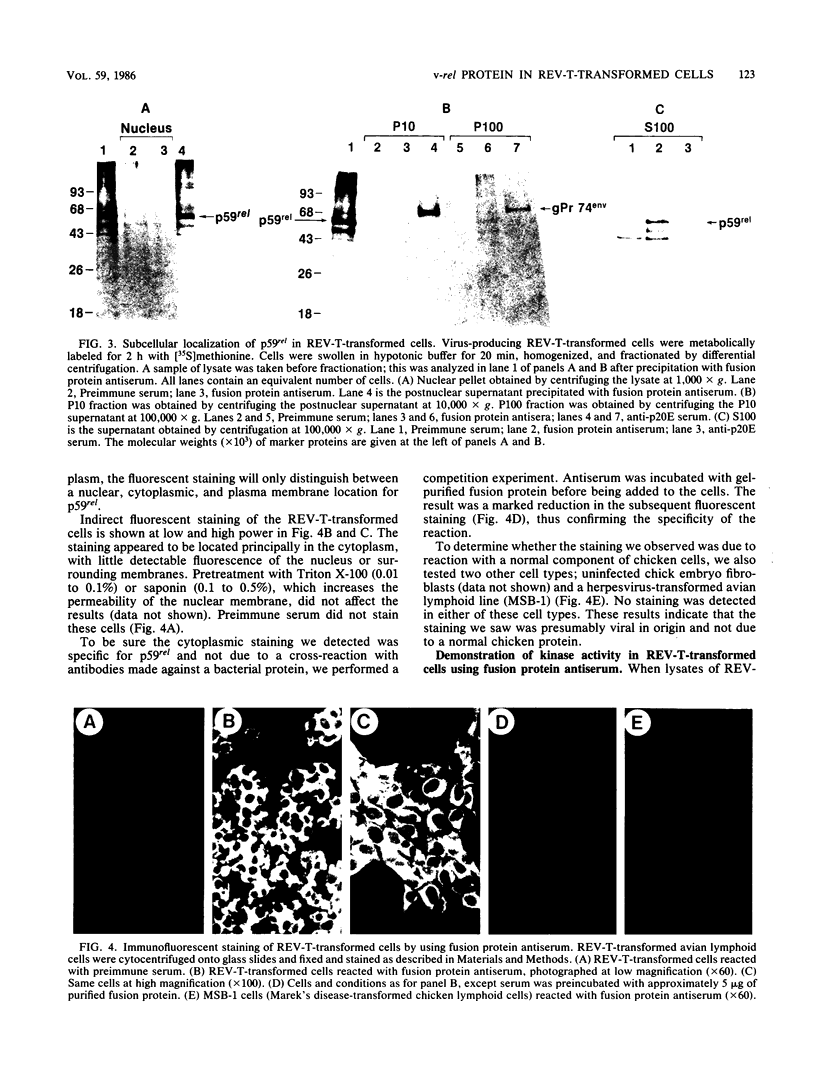
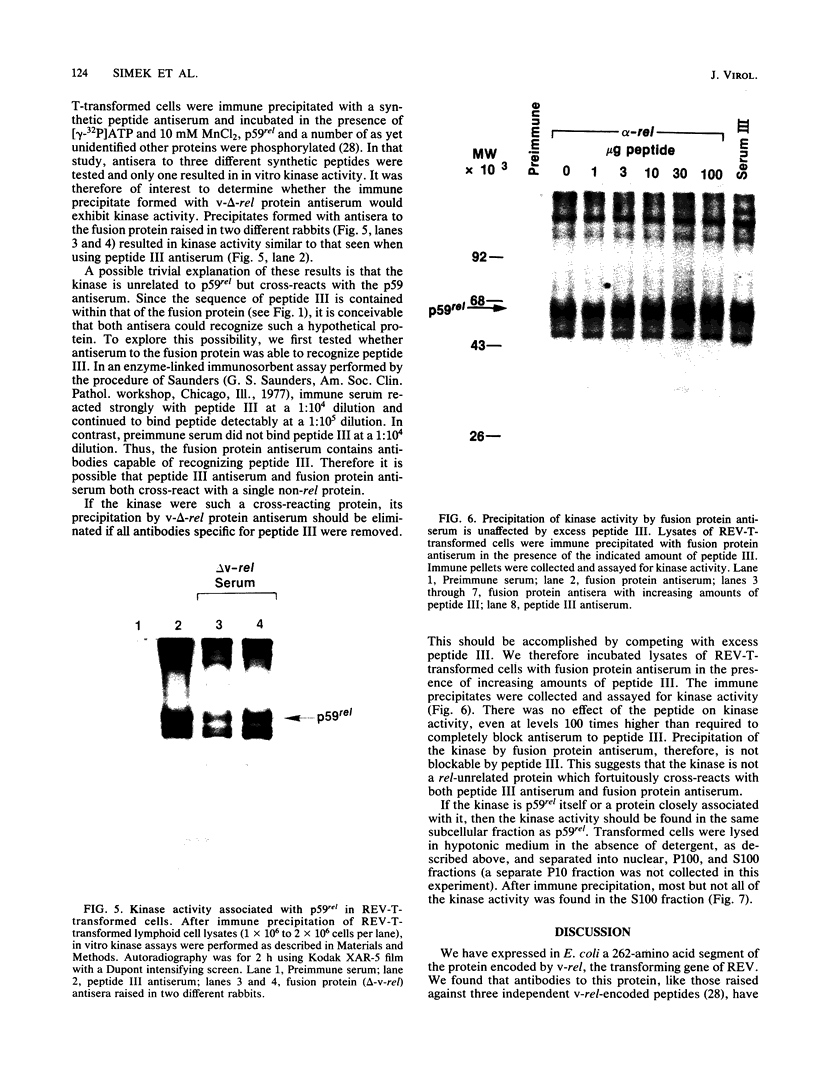
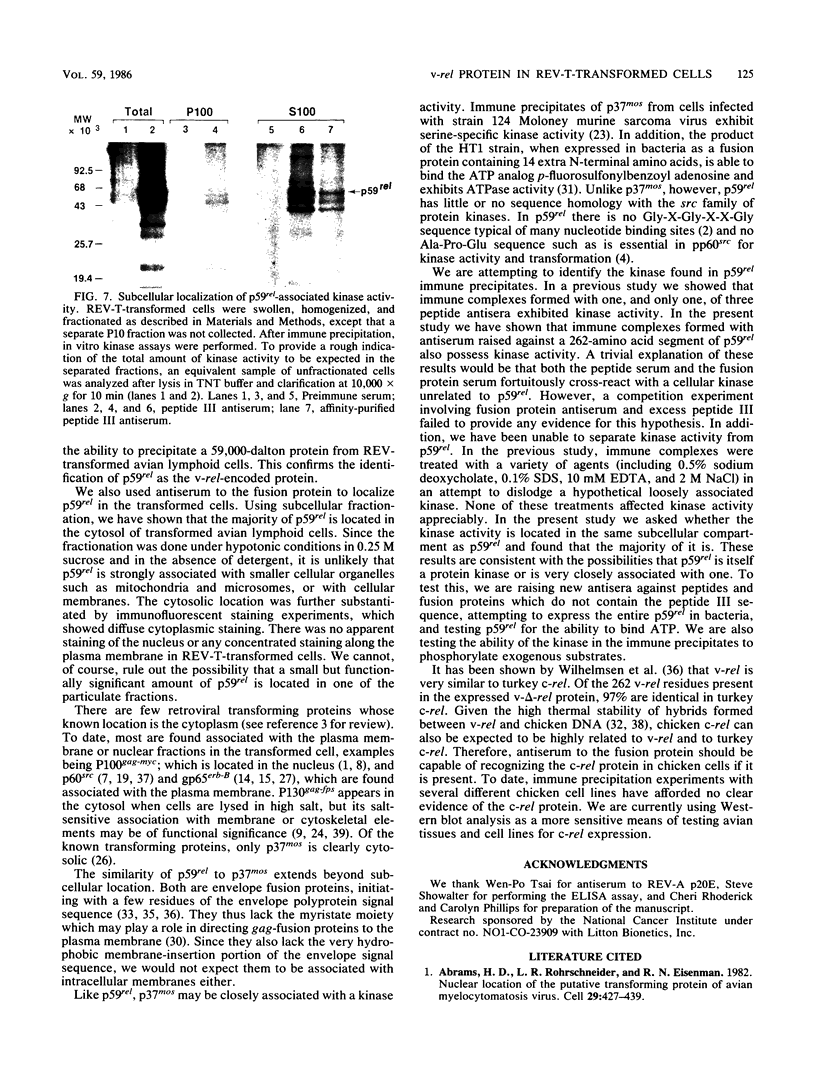
The protein (p59rel) encoded by the transforming gene of reticuloendotheliosis virus strain T (REV-T) has been identified in REV-T-transformed avian lymphoid cells by using antisera raised against synthetic peptides whose sequences were derived from three nonoverlapping regions of v-rel (N. R. Rice, T. D. Copeland, S. Simek, S. Oroszlan, and R. V. Gilden, Virology 149:217-229, 1986). To obtain polyclonal antibodies directed against a larger number of p59rel epitopes, a 262-amino acid segment was expressed in bacteria. Antisera raised against this fusion protein (v-delta-rel) precipitated p59rel from lysates of [35S]methionine-labeled REV-T-transformed cells, thus confirming previous results obtained with the peptide antisera. We used this new antiserum to localize p59rel in REV-T-transformed cells by subcellular fractionation using differential centrifugation and by indirect immune fluorescent staining. After fractionation and immune precipitation, the majority of p59rel was found in the cytosolic fraction. Indirect immunofluorescence experiments also gave results consistent with the cytoplasmic localization of the v-rel protein in transformed lymphoid cells. In previous studies (Rice et al., Virology 149:217-229, 1986) it was shown that immune precipitates formed with one of the three p59rel peptide antisera possessed in vitro protein kinase activity. Immune precipitates formed with the fusion protein antiserum also showed kinase activity in the in vitro assay. Most of this activity was found in the soluble cytoplasmic fraction, indicating that the kinase may be p59rel or a protein closely associated with it.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams H. D., Rohrschneider L. R., Eisenman R. N. Nuclear location of the putative transforming protein of avian myelocytomatosis virus. Cell. 1982 Jun;29(2):427–439. doi: 10.1016/0092-8674(82)90159-3. [DOI] [PubMed] [Google Scholar]
- Barker W. C., Dayhoff M. O. Viral src gene products are related to the catalytic chain of mammalian cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 May;79(9):2836–2839. doi: 10.1073/pnas.79.9.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. L., Parsons J. T. Amino acid alterations within a highly conserved region of the Rous sarcoma virus src gene product pp60src inactivate tyrosine protein kinase activity. Mol Cell Biol. 1984 May;4(5):862–866. doi: 10.1128/mcb.4.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Mak T. W., O'Rear J. J., Temin H. M. Characterization of reticuloendotheliosis virus strain T DNA and isolation of a novel variant of reticuloendotheliosis virus strain T by molecular cloning. J Virol. 1981 Dec;40(3):800–811. doi: 10.1128/jvi.40.3.800-811.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Wilhelmsen K. C., Temin H. M. Structure and expression of c-rel, the cellular homolog to the oncogene of reticuloendotheliosis virus strain T. J Virol. 1983 Jan;45(1):104–113. doi: 10.1128/jvi.45.1.104-113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner P., Greiser-Wilke I., Moelling K. Nuclear localization and DNA binding of the transforming gene product of avian myelocytomatosis virus. Nature. 1982 Mar 18;296(5854):262–269. doi: 10.1038/296262a0. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Wang E., Hanafusa H. Cytoplasmic localization of the transforming protein of Fujinami sarcoma virus: salt-sensitive association with subcellular components. J Virol. 1983 Feb;45(2):782–791. doi: 10.1128/jvi.45.2.782-791.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting T., Kabat D. Evidence for a glycoprotein "signal" involved in transport between subcellular organelles. Two membrane glycoproteins encoded by murine leukemia virus reach the cell surface at different rates. J Biol Chem. 1982 Dec 10;257(23):14011–14017. [PubMed] [Google Scholar]
- Franklin R. B., Maldonado R. L., Bose H. R. Isolation and characterization of reticuloendotheliosis virus transformed bone marrow cells. Intervirology. 1974;3(5-6):342–352. doi: 10.1159/000149771. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Rice N. R., Gilden R. V. Avian reticuloendotheliosis virus: characterization of the high-molecular-weight viral RNA in transforming and helper virus populations. J Virol. 1980 Jun;34(3):743–751. doi: 10.1128/jvi.34.3.743-751.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J. Studies on the formation of the influenza virus envelope. Virology. 1974 Aug;60(2):398–418. doi: 10.1016/0042-6822(74)90335-3. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Beug H. Identification of a form of the avian erythroblastosis virus erb-B gene product at the cell surface. 1984 May 31-Jun 6Nature. 309(5967):460–462. doi: 10.1038/309460a0. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Ramsay G. M., Savin K., Kitchener G., Graf T., Beug H. Identification and characterization of the avian erythroblastosis virus erbB gene product as a membrane glycoprotein. Cell. 1983 Feb;32(2):579–588. doi: 10.1016/0092-8674(83)90477-4. [DOI] [PubMed] [Google Scholar]
- Hoelzer J. D., Franklin R. B., Bose H. R., Jr Transformation by reticuloendotheliosis virus: development of a focus assay and isolation of a nontransforming virus. Virology. 1979 Feb;93(1):20–30. doi: 10.1016/0042-6822(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Hoelzer J. D., Lewis R. B., Wasmuth C. R., Bose H. R., Jr Hematopoietic cell transformation by reticuloendotheliosis virus: characterization of the genetic defect. Virology. 1980 Jan 30;100(2):462–474. doi: 10.1016/0042-6822(80)90536-x. [DOI] [PubMed] [Google Scholar]
- Hu S. S., Lai M. M., Wong T. C., Cohen R. S., Sevoian M. Avian reticuloendotheliosis virus: characterization of genome structure by heteroduplex mapping. J Virol. 1981 Mar;37(3):899–907. doi: 10.1128/jvi.37.3.899-907.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R. A., Mitchell R. L., Lau A. F., Faras A. J. Association of pp60src and src protein kinase activity with the plasma membrane of nonpermissive and permissive avian sarcoma virus-infected cells. J Virol. 1980 Dec;36(3):805–815. doi: 10.1128/jvi.36.3.805-815.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lautenberger J. A., Court D., Papas T. S. High-level expression in Escherichia coli of the carboxy-terminal sequences of the avian myelocytomatosis virus (MC29) v-myc protein. Gene. 1983 Jul;23(1):75–84. doi: 10.1016/0378-1119(83)90218-4. [DOI] [PubMed] [Google Scholar]
- Lyles D. S., McConnell K. A. Subcellular localization of the env-related glycoproteins in Friend erythroleukemia cells. J Virol. 1981 Jul;39(1):263–272. doi: 10.1128/jvi.39.1.263-272.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S. A., Arlinghaus R. B. Serine kinase activity associated with Maloney murine sarcoma virus-124-encoded p37mos. Virology. 1985 May;143(1):321–333. doi: 10.1016/0042-6822(85)90119-9. [DOI] [PubMed] [Google Scholar]
- Moss P., Radke K., Carter V. C., Young J., Gilmore T., Martin G. S. Cellular localization of the transforming protein of wild-type and temperature-sensitive Fujinami sarcoma virus. J Virol. 1984 Nov;52(2):557–565. doi: 10.1128/jvi.52.2.557-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Walter G., Singer S. J. On the nature of crossreactions observed with antibodies directed to defined epitopes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5939–5943. doi: 10.1073/pnas.79.19.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Nigg E. A., Hunter T. The transforming protein of Moloney murine sarcoma virus is a soluble cytoplasmic protein. Cell. 1983 May;33(1):161–172. doi: 10.1016/0092-8674(83)90345-8. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L., Bishop J. M. Subcellular localization of the v-erb-B protein, the product of a transforming gene of avian erythroblastosis virus. Virology. 1984 Jun;135(2):356–368. doi: 10.1016/0042-6822(84)90192-2. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Copeland T. D., Simek S., Oroszlan S., Gilden R. V. Detection and characterization of the protein encoded by the v-rel oncogene. Virology. 1986 Mar;149(2):217–229. doi: 10.1016/0042-6822(86)90123-6. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Hiebsch R. R., Gonda M. A., Bose H. R., Jr, Gilden R. V. Genome of reticuloendotheliosis virus: characterization by use of cloned proviral DNA. J Virol. 1982 Apr;42(1):237–252. doi: 10.1128/jvi.42.1.237-252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A., Oroszlan S. Myristylation of gag-onc fusion proteins in mammalian transforming retroviruses. Virology. 1984 Mar;133(2):431–437. doi: 10.1016/0042-6822(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Seth A., Vande Woude G. F. Nucleotide sequence and biochemical activities of the Moloney murine sarcoma virus strain HT-1 mos gene. J Virol. 1985 Oct;56(1):144–152. doi: 10.1128/jvi.56.1.144-152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek S., Rice N. R. Analysis of the nucleic acid components in reticuloendotheliosis virus. J Virol. 1980 Jan;33(1):320–329. doi: 10.1128/jvi.33.1.320-329.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. M., Rice N. R., Hiebsch R. R., Bose H. R., Jr, Gilden R. V. Nucleotide sequence of v-rel: the oncogene of reticuloendotheliosis virus. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6229–6233. doi: 10.1073/pnas.80.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W. P., Copeland T. D., Oroszlan S. Purification and chemical and immunological characterization of avian reticuloendotheliosis virus gag-gene-encoded structural proteins. Virology. 1985 Jan 30;140(2):289–312. doi: 10.1016/0042-6822(85)90367-8. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Galleshaw J. A., Jonas V., Berns A. J., Doolittle R. F., Donoghue D. J., Verma I. M. Nucleotide sequence and formation of the transforming gene of a mouse sarcoma virus. Nature. 1981 Jan 22;289(5795):258–262. doi: 10.1038/289258a0. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen K. C., Eggleton K., Temin H. M. Nucleic acid sequences of the oncogene v-rel in reticuloendotheliosis virus strain T and its cellular homolog, the proto-oncogene c-rel. J Virol. 1984 Oct;52(1):172–182. doi: 10.1128/jvi.52.1.172-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Jay G., Pastan I. Localization of the ASV src gene product to the plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1979 Sep;18(1):125–134. doi: 10.1016/0092-8674(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Wong T. C., Lai M. M. Avian reticuloendotheliosis virus contains a new class of oncogene of turkey origin. Virology. 1981 May;111(1):289–293. doi: 10.1016/0042-6822(81)90674-7. [DOI] [PubMed] [Google Scholar]
- Woolford J., Beemon K. Transforming proteins of fujinami and PRCII avian sarcoma viruses have different subcellular locations. Virology. 1984 May;135(1):168–180. doi: 10.1016/0042-6822(84)90127-2. [DOI] [PubMed] [Google Scholar]