Abstract
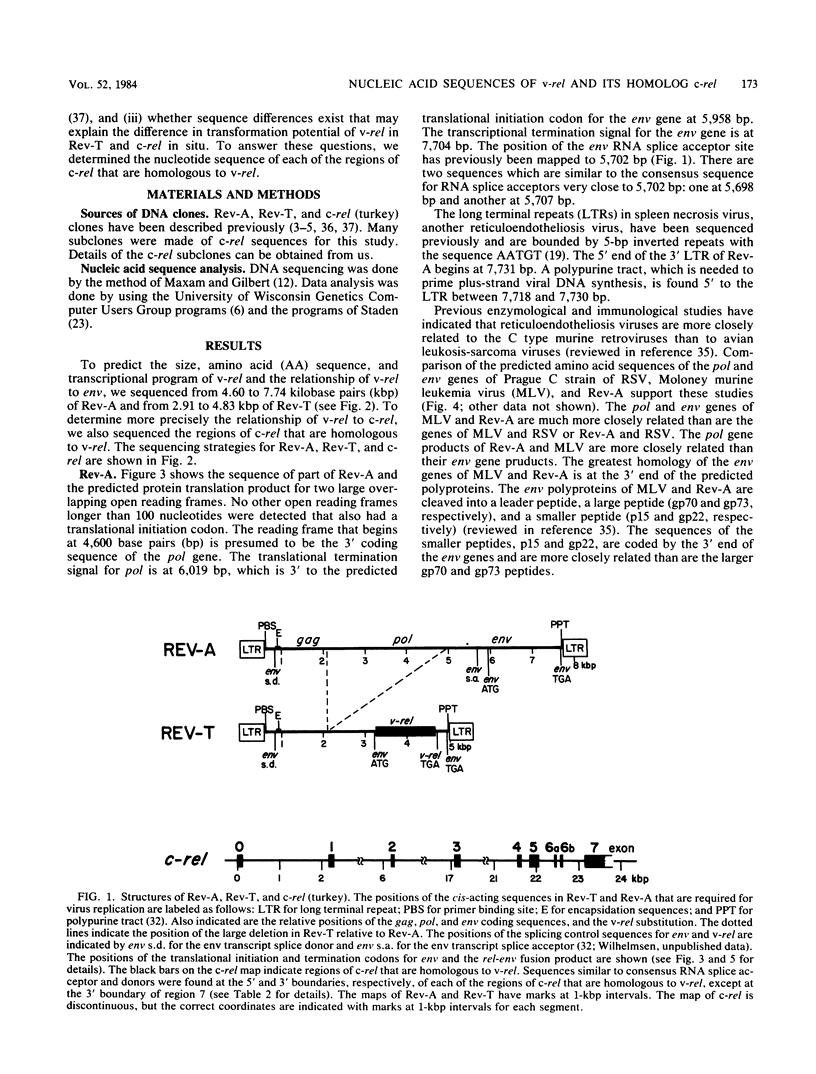
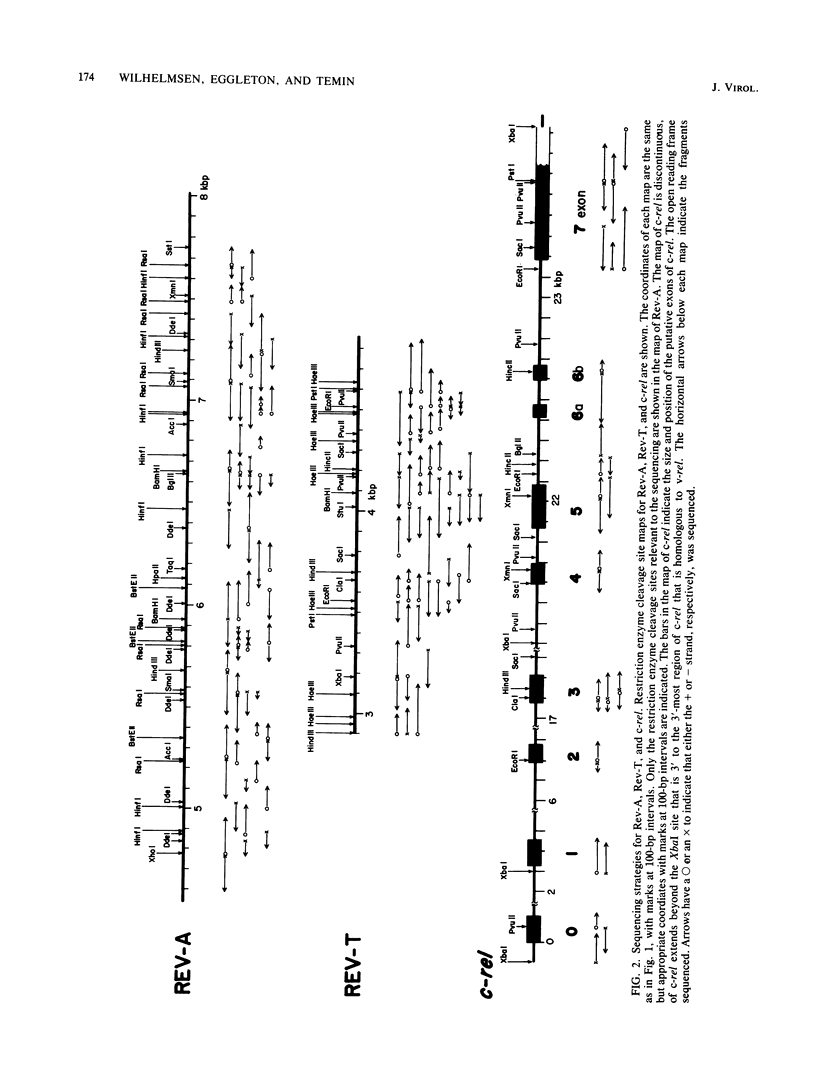
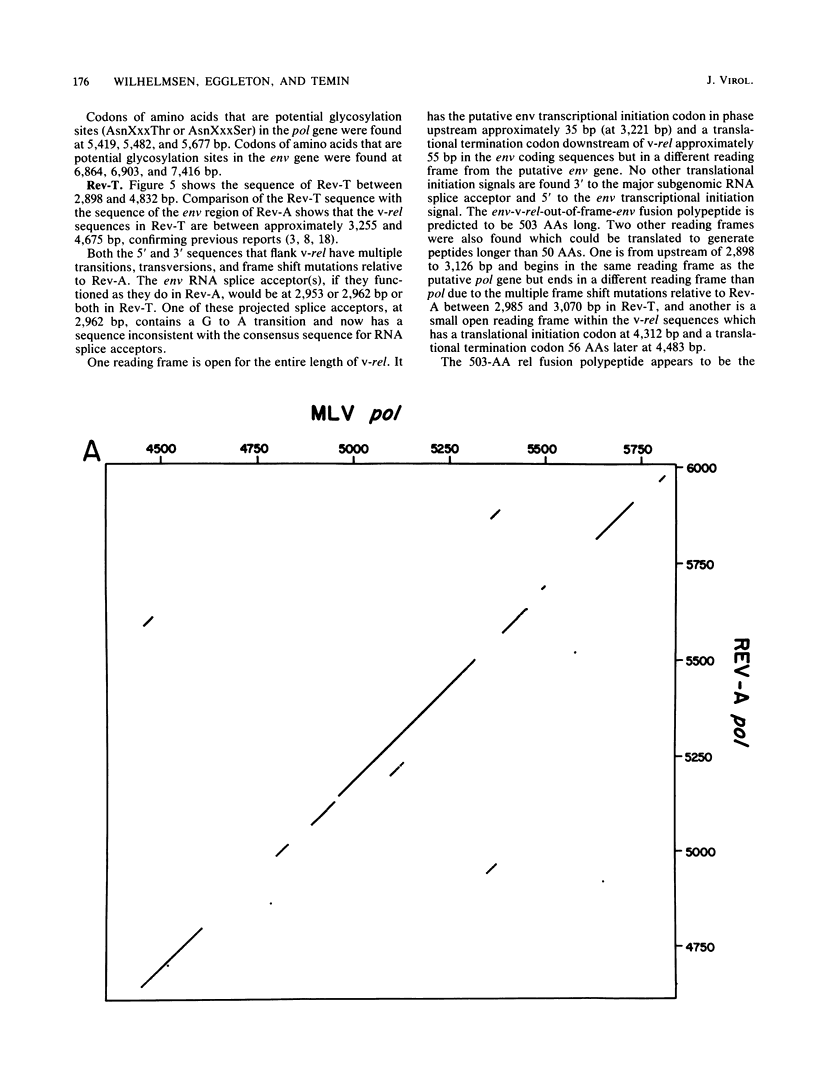
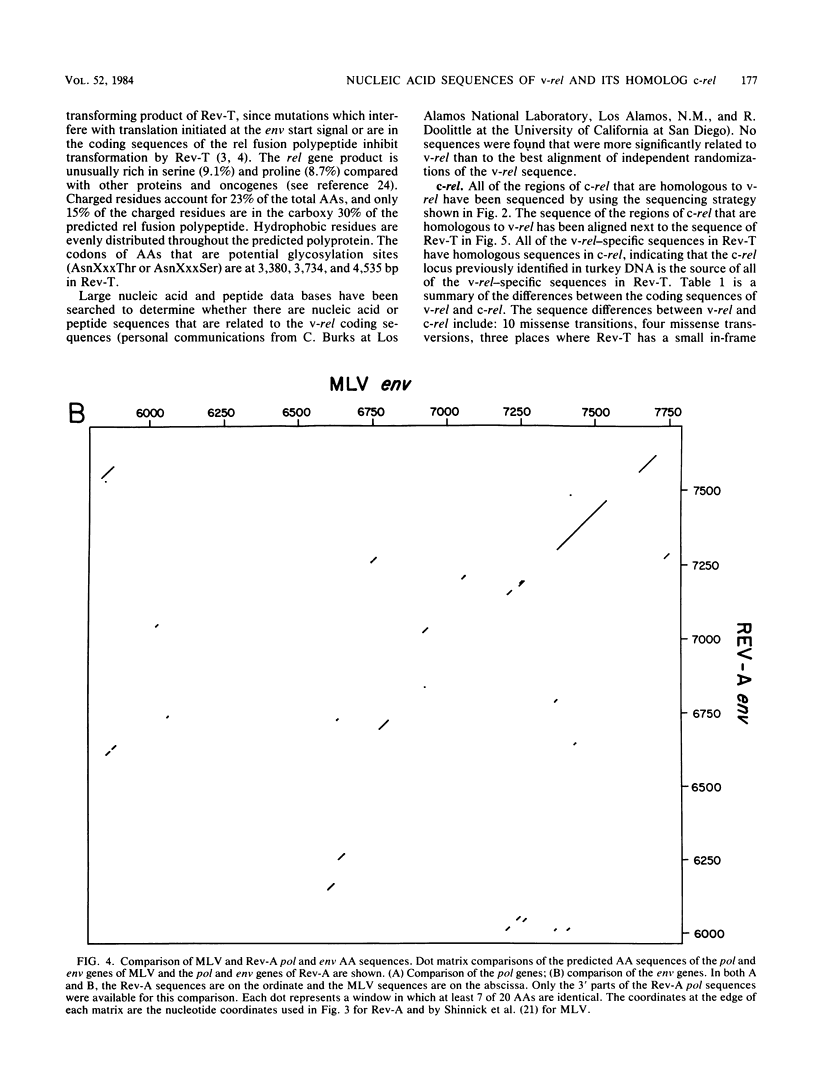
Reticuloendotheliosis virus strain T (Rev-T) is a highly oncogenic replication-defective retrovirus which contains the oncogene v-rel. It is thought that Rev-T arose when a virus similar to Rev-A, the helper virus of Rev-T, infected a turkey and recombined with c-rel from that turkey. There is one large c-rel locus in the turkey genome which contains all of the sequences homologous to v-rel (K. C. Wilhelmsen and H. M. Temin, J. Virol. 49:521-529, 1984). We have sequenced v-rel and its flanking sequences, each of the regions of the c-rel locus from turkey that are homologous to v-rel and their flanking sequences, and the coding sequence for env and part of pol of Rev-A. The v-rel coding sequences can be translated into a 503-amino acid env-v-rel-out-of-frame-env fusion polypeptide. We have not detected any sequences in the Los Alamos or University of California-San Diego data bases that are more significantly related to the amino acid or nucleic acid sequence of v-rel than to the randomized sequence of v-rel. Comparison of Rev-A, Rev-T, and c-rel indicates that the v-rel sequences may have been transduced from the c-rel (turkey) locus by a novel mechanism. There are sequences in Rev-A and c-rel that are similar to splicing signals, indicating that the 5' virus-rel junction of Rev-T may have been formed by cellular RNA splicing machinery. Eight presumed introns have presumably been spliced out of c-rel to generate v-rel. There are also short imperfect regions of homology between sequences at the boundaries of v-rel and sequences in Rev-A and c-rel (turkey), indicating that c-rel may have been transduced by homologous recombination. There are many differences between the amino acid sequences of the predicted translational products of v-rel and c-rel which may account for their difference in transformation potential. These sequence differences between v-rel and c-rel include 10 missense transitions, four missense transversions, and three places where Rev-T has a small in-frame deletion of sequences relative to c-rel. Most of the coding sequence differences between c-rel and v-rel are nonconservative amino acid changes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer G., Temin H. M. Radioimmunological comparison of the DNA polymerases of avian retroviruses. J Virol. 1980 Mar;33(3):1046–1057. doi: 10.1128/jvi.33.3.1046-1057.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G., Temin H. M. Specific antigenic relationships between the RNA-dependent DNA polymerases of avian reticuloendotheliosis viruses and mammalian type C retroviruses. J Virol. 1980 Apr;34(1):168–177. doi: 10.1128/jvi.34.1.168-177.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Mak T. W., O'Rear J. J., Temin H. M. Characterization of reticuloendotheliosis virus strain T DNA and isolation of a novel variant of reticuloendotheliosis virus strain T by molecular cloning. J Virol. 1981 Dec;40(3):800–811. doi: 10.1128/jvi.40.3.800-811.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Temin H. M. Substitution of 5' helper virus sequences into non-rel portion of reticuloendotheliosis virus strain T suppresses transformation of chicken spleen cells. Cell. 1982 Nov;31(1):111–120. doi: 10.1016/0092-8674(82)90410-x. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Wilhelmsen K. C., Temin H. M. Structure and expression of c-rel, the cellular homolog to the oncogene of reticuloendotheliosis virus strain T. J Virol. 1983 Jan;45(1):104–113. doi: 10.1128/jvi.45.1.104-113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Hu S. S., Lai M. M., Wong T. C., Cohen R. S., Sevoian M. Avian reticuloendotheliosis virus: characterization of genome structure by heteroduplex mapping. J Virol. 1981 Mar;37(3):899–907. doi: 10.1128/jvi.37.3.899-907.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Kopchick J. J., Harless J., Geisser B. S., Killam R., Hewitt R. R., Arlinghaus R. B. Endodeoxyribonuclease activity associated with Rauscher murine leukemia virus. J Virol. 1981 Jan;37(1):274–283. doi: 10.1128/jvi.37.1.274-283.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. G., Glover C. Low-molecular-weight RNAs and initiation of RNA-directed DNA synthesis in avian reticuloendotheliosis virus. J Virol. 1980 Feb;33(2):708–716. doi: 10.1128/jvi.33.2.708-716.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Watson D. K., Schultz R. A., Lautenberger J., Papas T. S. Nucleotide sequence analysis of the proviral genome of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1983 May;80(9):2500–2504. doi: 10.1073/pnas.80.9.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Aaronson S. A. Complete nucleotide sequence and organization of the Moloney murine sarcoma virus genome. Science. 1981 Oct 23;214(4519):445–450. doi: 10.1126/science.6170110. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Hiebsch R. R., Gonda M. A., Bose H. R., Jr, Gilden R. V. Genome of reticuloendotheliosis virus: characterization by use of cloned proviral DNA. J Virol. 1982 Apr;42(1):237–252. doi: 10.1128/jvi.42.1.237-252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Temin H. M. Loss of intervening sequences in genomic mouse alpha-globin DNA inserted in an infectious retrovirus vector. Nature. 1982 Sep 16;299(5880):265–268. doi: 10.1038/299265a0. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Sorge J., Hughes S. H. Splicing of intervening sequences introduced into an infectious retroviral vector. J Mol Appl Genet. 1982;1(6):547–559. [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. M., Rice N. R., Hiebsch R. R., Bose H. R., Jr, Gilden R. V. Nucleotide sequence of v-rel: the oncogene of reticuloendotheliosis virus. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6229–6233. doi: 10.1073/pnas.80.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Theilen G. H., Zeigel R. F., Twiehaus M. J. Biological studies with RE virus (strain T) that induces reticuloendotheliosis in turkeys, chickens, and Japanese quail. J Natl Cancer Inst. 1966 Dec;37(6):731–743. [PubMed] [Google Scholar]
- Van Beveren C., Galleshaw J. A., Jonas V., Berns A. J., Doolittle R. F., Donoghue D. J., Verma I. M. Nucleotide sequence and formation of the transforming gene of a mouse sarcoma virus. Nature. 1981 Jan 22;289(5795):258–262. doi: 10.1038/289258a0. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Temin H. M. Construction of a helper cell line for avian reticuloendotheliosis virus cloning vectors. Mol Cell Biol. 1983 Dec;3(12):2241–2249. doi: 10.1128/mcb.3.12.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. C. Transfer RNAs associated with the 70S RNA of AKR murine leukemia virus. Biochem Biophys Res Commun. 1975 Aug 4;65(3):1130–1136. doi: 10.1016/s0006-291x(75)80503-1. [DOI] [PubMed] [Google Scholar]
- Watson D. K., Reddy E. P., Duesberg P. H., Papas T. S. Nucleotide sequence analysis of the chicken c-myc gene reveals homologous and unique coding regions by comparison with the transforming gene of avian myelocytomatosis virus MC29, delta gag-myc. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2146–2150. doi: 10.1073/pnas.80.8.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K. C., Chen I. S., Temin H. M. The organization of c-rel in chicken and turkey DNAS. Prog Clin Biol Res. 1983;119:43–56. [PubMed] [Google Scholar]
- Wilhelmsen K. C., Temin H. M. Structure and dimorphism of c-rel (turkey), the cellular homolog to the oncogene of reticuloendotheliosis virus strain T. J Virol. 1984 Feb;49(2):521–529. doi: 10.1128/jvi.49.2.521-529.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


