Abstract
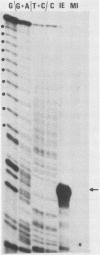
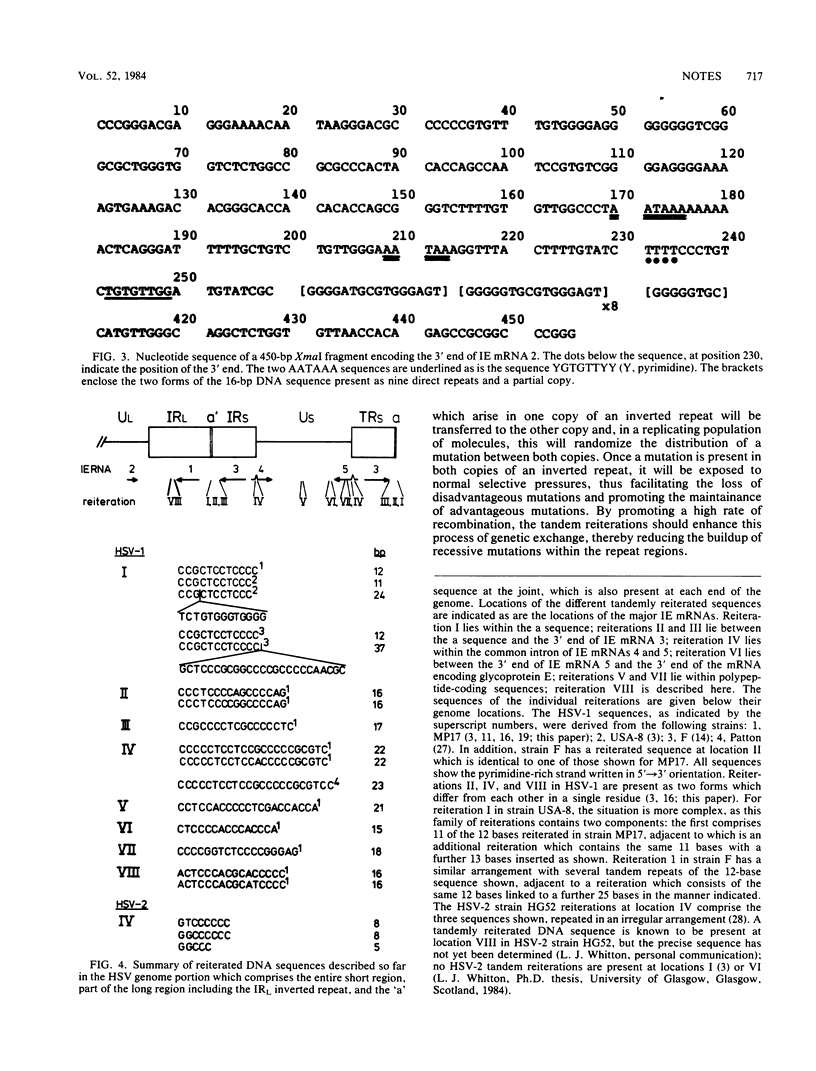
The 3' end of immediate-early mRNA 1 was mapped precisely within the IRL/TRL genome regions, and the DNA sequences around the 3' end were determined. An AATAAA polyadenylation signal was present 17 base pairs upstream of the 3' end, and eight tandemly repeated copies of a 16-base-pair sequence (GGGGGTGCGTGGGAGT) plus one further closely related copy were located 20 base pairs downstream. Other tandem reiterations present in the herpes simplex virus genome are described and their properties are considered.
Full text
PDF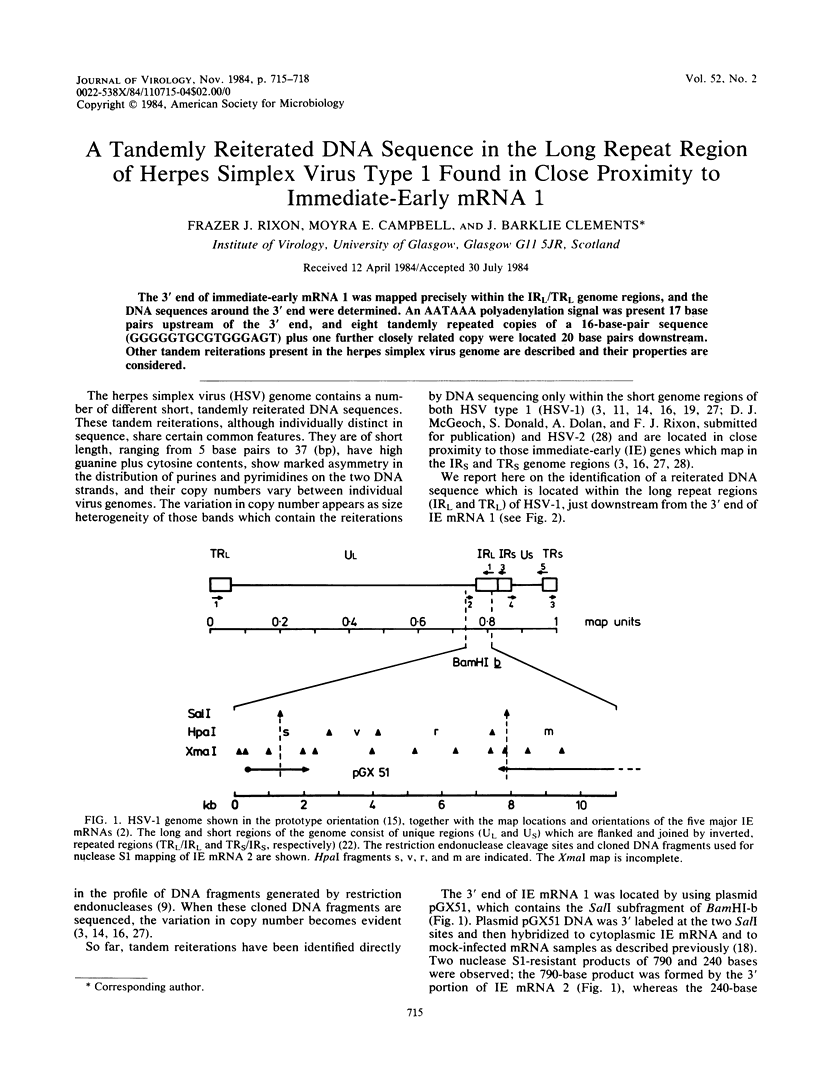


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Selby M. J., Rutter W. J. The highly polymorphic region near the human insulin gene is composed of simple tandemly repeating sequences. Nature. 1982 Jan 7;295(5844):31–35. doi: 10.1038/295031a0. [DOI] [PubMed] [Google Scholar]
- Clements J. B., McLauchlan J., McGeoch D. J. Orientation of herpes simplex virus type 1 immediate early mRNA's. Nucleic Acids Res. 1979 Sep 11;7(1):77–91. doi: 10.1093/nar/7.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Nucleotide sequences of the joint between the L and S segments of herpes simplex virus types 1 and 2. J Gen Virol. 1981 Aug;55(Pt 2):315–331. doi: 10.1099/0022-1317-55-2-315. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Garfinkel M. D., Pruitt R. E., Meyerowitz E. M. DNA sequences, gene regulation and modular protein evolution in the Drosophila 68C glue gene cluster. J Mol Biol. 1983 Aug 25;168(4):765–789. doi: 10.1016/s0022-2836(83)80074-6. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Ellis J., Svec P., Schlesinger D. H., Nussenzweig V. Identification and chemical synthesis of a tandemly repeated immunogenic region of Plasmodium knowlesi circumsporozoite protein. Nature. 1983 Sep 1;305(5929):29–33. doi: 10.1038/305029a0. [DOI] [PubMed] [Google Scholar]
- Heilig R., Muraskowsky R., Mandel J. L. The ovalbumin gene family. The 5' end region of the X and Y genes. J Mol Biol. 1982 Mar 25;156(1):1–19. doi: 10.1016/0022-2836(82)90455-7. [DOI] [PubMed] [Google Scholar]
- Lebo R. V., Chakravarti A., Buetow K. H., Cheung M. C., Cann H., Cordell B., Goodman H. Recombination within and between the human insulin and beta-globin gene loci. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4808–4812. doi: 10.1073/pnas.80.15.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Brown S. M., Lang J., Subak-Sharpe J. H., Koprowski H., Warren K. G. Variations in herpes simplex virus isolated from human ganglia and a study of clonal variation in HSV-1. Ann N Y Acad Sci. 1980;354:291–308. doi: 10.1111/j.1749-6632.1980.tb27973.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McLauchlan J., Clements J. B. DNA sequence homology between two co-linear loci on the HSV genome which have different transforming abilities. EMBO J. 1983;2(11):1953–1961. doi: 10.1002/j.1460-2075.1983.tb01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Roizman B. Site-specific inversion sequence of the herpes simplex virus genome: domain and structural features. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7047–7051. doi: 10.1073/pnas.78.11.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Buchman T. G., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus DNA. IX. Apparent exclusion of some parental DNA arrangements in the generation of intertypic (HSV-1 X HSV-2) recombinants. J Virol. 1977 Oct;24(1):231–248. doi: 10.1128/jvi.24.1.231-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie M. J., McGeoch D. J. DNA sequence analysis of an immediate-early gene region of the herpes simplex virus type 1 genome (map coordinates 0.950 to 0.978). J Gen Virol. 1982 Sep;62(Pt 1):1–15. doi: 10.1099/0022-1317-62-1-1. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Nakai S., Honjo T. Switch region of immunoglobulin Cmu gene is composed of simple tandem repetitive sequences. Nature. 1981 Aug 27;292(5826):845–848. doi: 10.1038/292845a0. [DOI] [PubMed] [Google Scholar]
- Rixon F. J., Clements J. B. Detailed structural analysis of two spliced HSV-1 immediate-early mRNAs. Nucleic Acids Res. 1982 Apr 10;10(7):2241–2256. doi: 10.1093/nar/10.7.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon F. J., McGeoch D. J. A 3' co-terminal family of mRNAs from the herpes simplex virus type 1 short region: two overlapping reading frames encode unrelated polypeptide one of which has highly reiterated amino acid sequence. Nucleic Acids Res. 1984 Mar 12;12(5):2473–2487. doi: 10.1093/nar/12.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. BKV splice sequences based on analysis of preferred donor and acceptor sites. Nucleic Acids Res. 1979 Jul 25;6(10):3387–3398. doi: 10.1093/nar/6.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Sturtevant A H. The Effects of Unequal Crossing over at the Bar Locus in Drosophila. Genetics. 1925 Mar;10(2):117–147. doi: 10.1093/genetics/10.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Renz M. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 1984 May 25;12(10):4127–4138. doi: 10.1093/nar/12.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Umene K., Enquist L. W. Reiterated sequences within the intron of an immediate-early gene of herpes simplex virus type 1. Nucleic Acids Res. 1981 Aug 25;9(16):4189–4199. doi: 10.1093/nar/9.16.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. L., Clements J. B. The junctions between the repetitive and the short unique sequences of the herpes simplex virus genome are determined by the polypeptide-coding regions of two spliced immediate-early mRNAs. J Gen Virol. 1984 Mar;65(Pt 3):451–466. doi: 10.1099/0022-1317-65-3-451. [DOI] [PubMed] [Google Scholar]
- Whitton J. L., Rixon F. J., Easton A. J., Clements J. B. Immediate-early mRNA-2 of herpes simplex viruses types 1 and 2 is unspliced: conserved sequences around the 5' and 3' termini correspond to transcription regulatory signals. Nucleic Acids Res. 1983 Sep 24;11(18):6271–6287. doi: 10.1093/nar/11.18.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]