Abstract
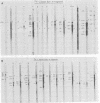
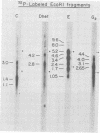
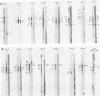
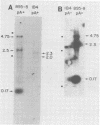
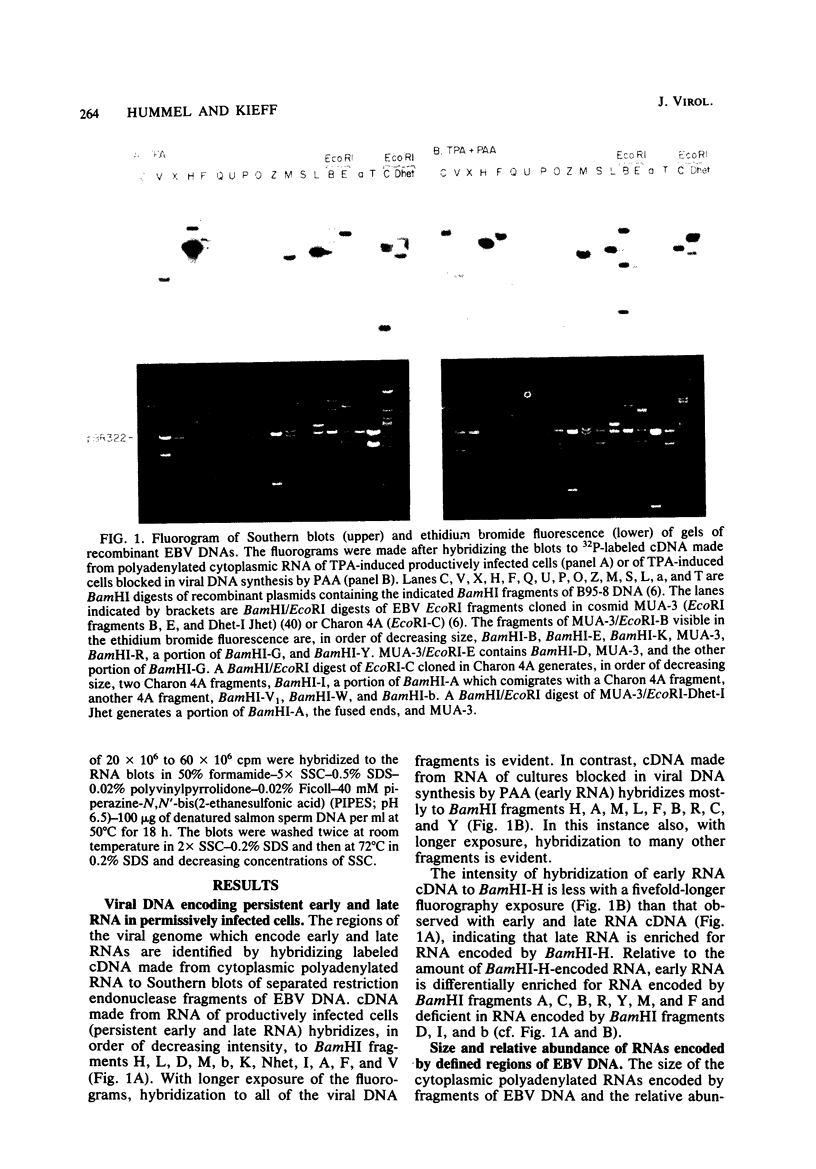
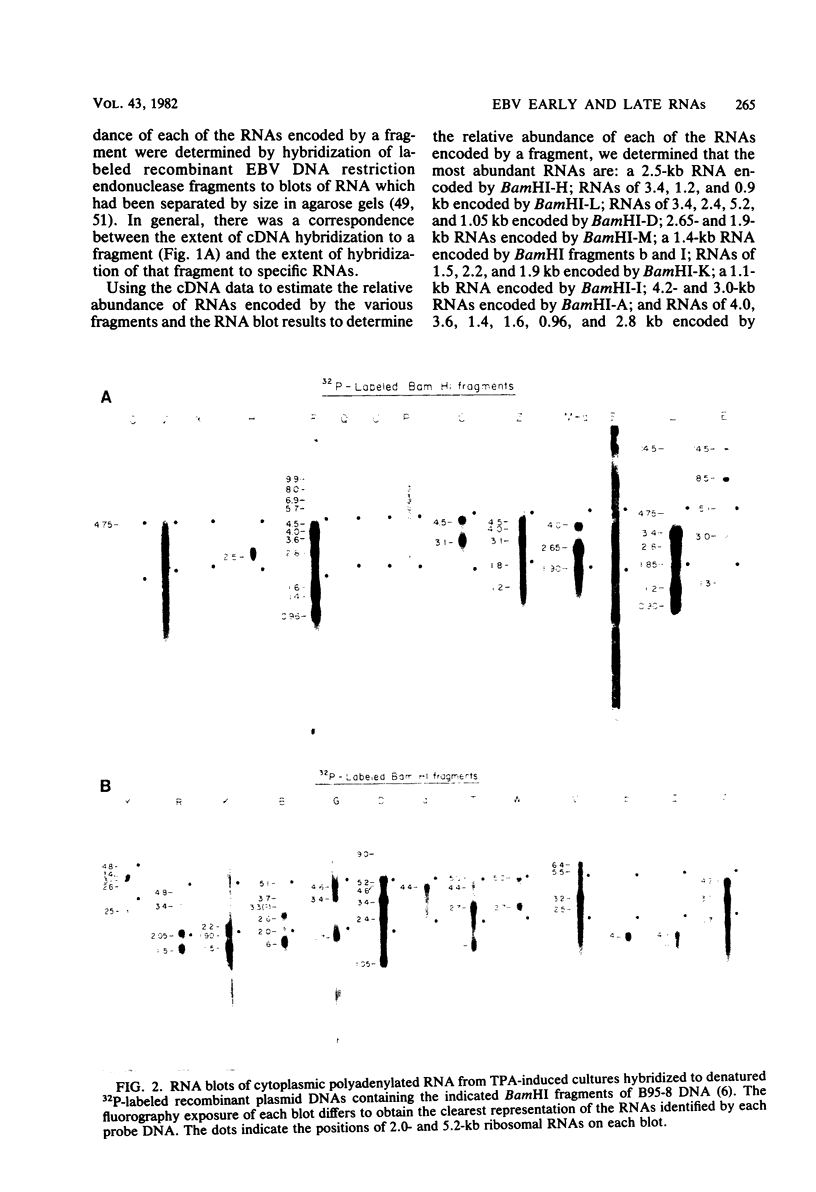
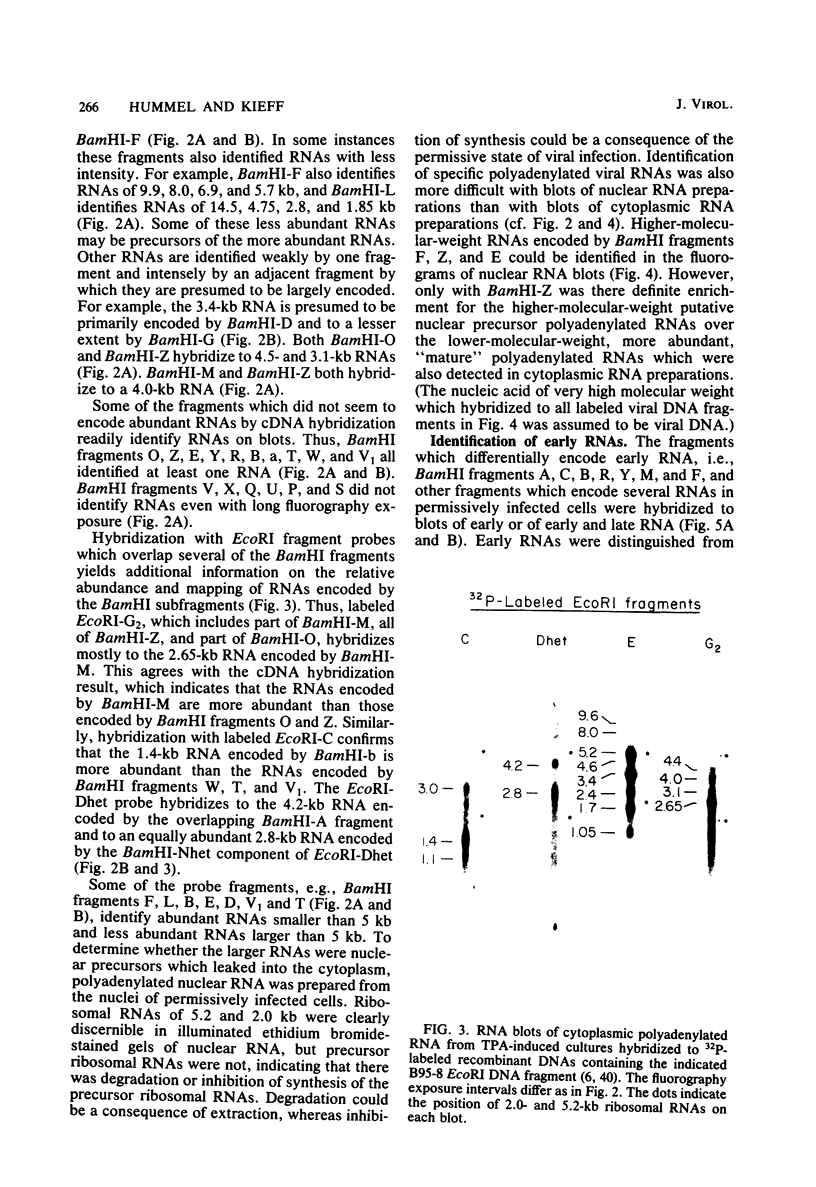
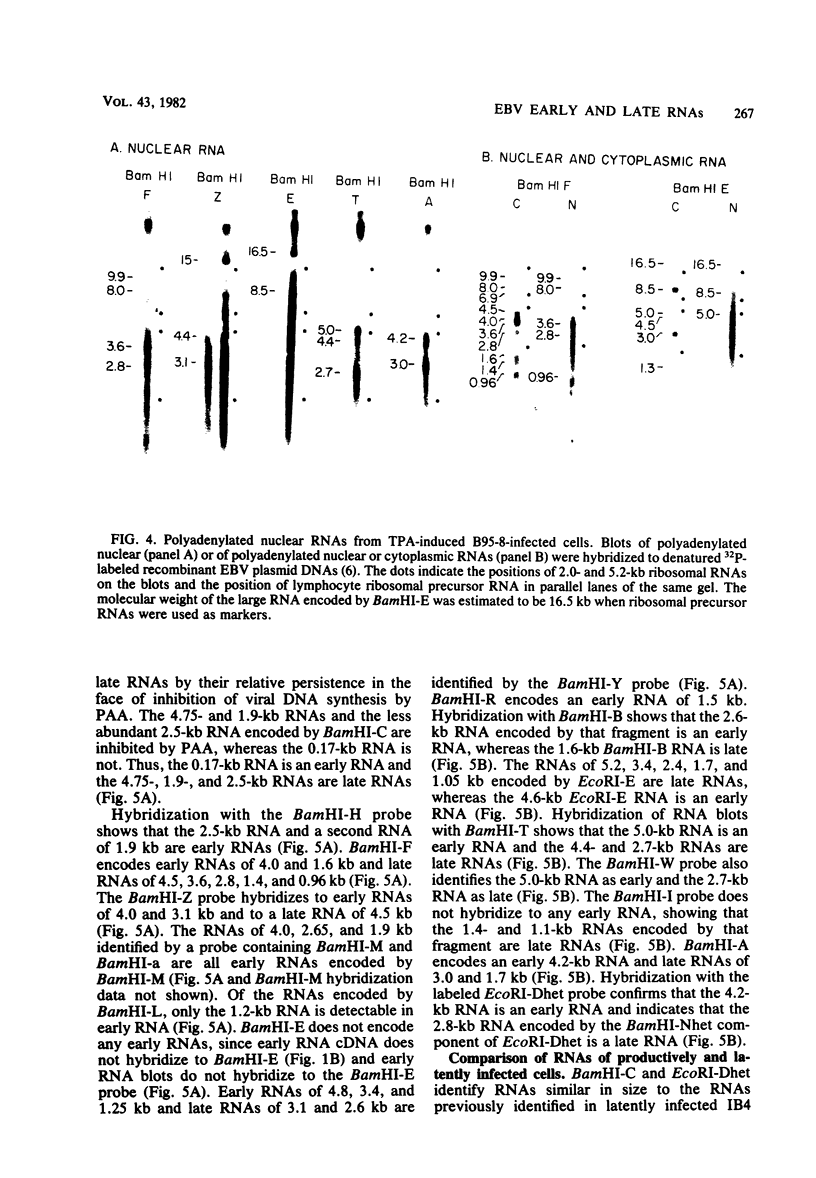
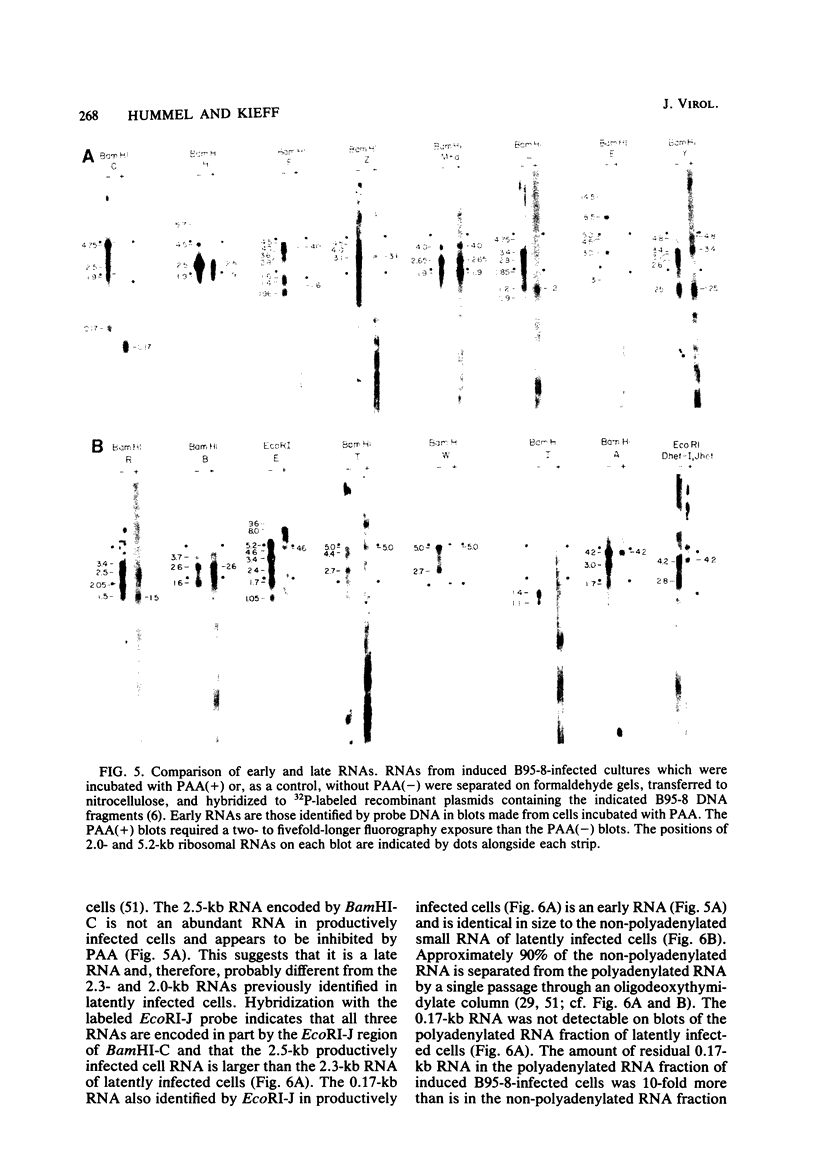
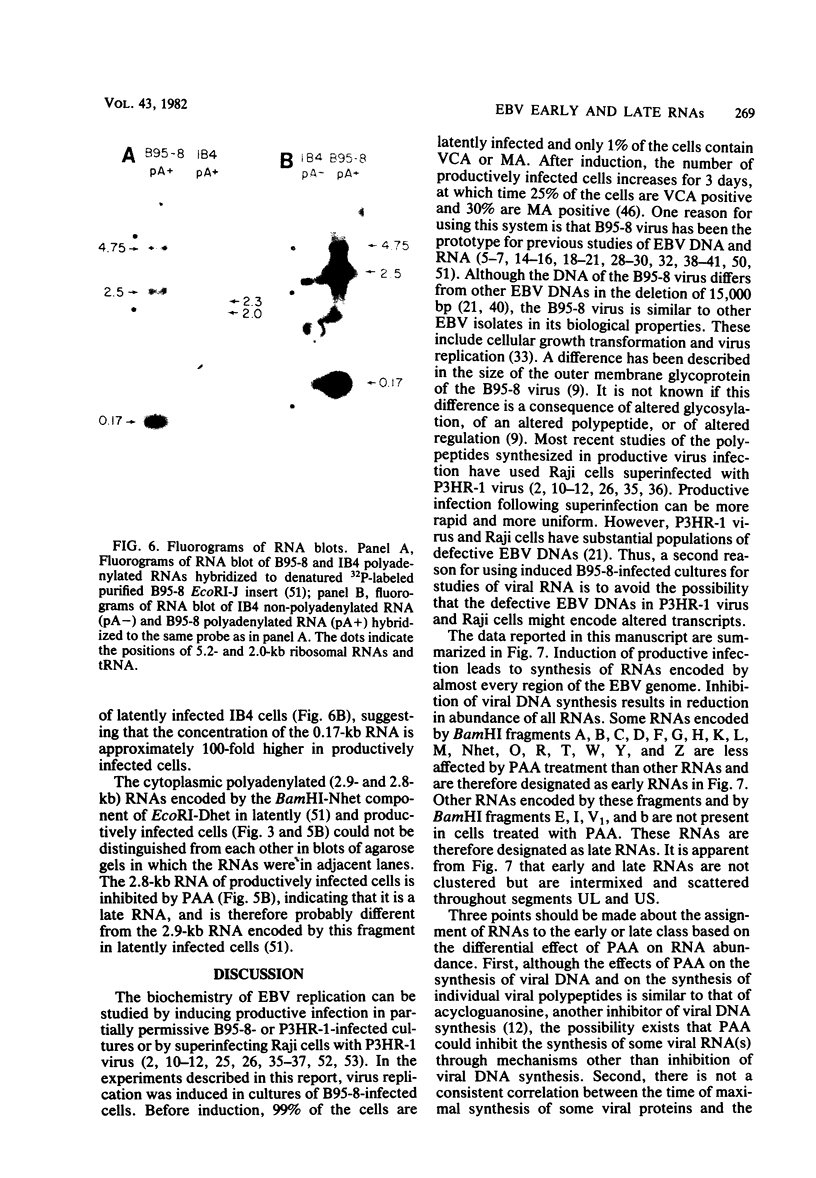
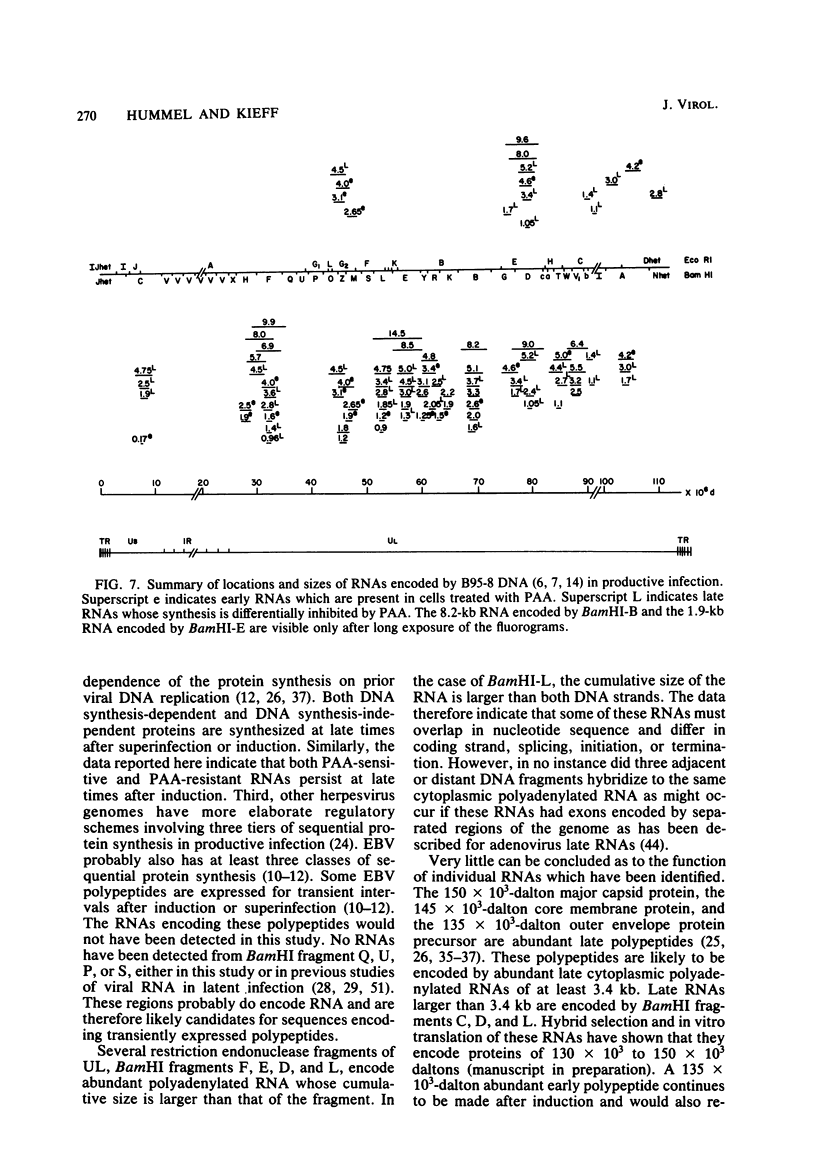
More than 50 RNAs expressed by Epstein-Barr virus late in productive infection have been identified. B95-8-infected cells were induced to a relatively high level of permissive infection with the tumor promotor 12-O-tetradecanoylphorbol-13-acetate. Polyadenylated RNAs were extracted from the cell cytoplasm, separated by size on formaldehyde gels, transferred to nitrocellulose, and hybridized to labeled recombinant Epstein-Barr virus DNA fragments. Comparison of RNAs from induced cultures with RNAs from induced cultures also treated with phosphonoacetic acid to inhibit viral DNA synthesis identifies two RNA classes: a persistent early class of RNAs whose abundance is relatively resistant to viral DNA synthesis inhibition and a late class of RNAs whose abundance is relatively sensitive to viral DNA synthesis inhibition. The persistent early and late RNAs are not clustered but are intermixed and scattered through most of segments UL and US. The cytoplasmic polyadenylated RNAs expressed during latent infection were not detected in productively infected cells, indicating that different classes of viral RNA are associated with latent and productive infection. Non-polyadenylated small RNAs originally identified in cells latently infected with Epstein-Barr virus are expressed in greater abundance in productively infected cells and are part of the early RNA class.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss G. J., Nonoyama M. Mechanisms of infection with Epstein-Barr virus. III. The synthesis of proteins in superinfected Raji cells. Virology. 1978 Jun 1;87(1):204–207. doi: 10.1016/0042-6822(78)90173-3. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Zimber U., Hudewentz J., Epstein M. A. Comparison of Epstein-Barr virus strains of different origin by analysis of the viral DNAs. J Virol. 1980 Sep;35(3):603–618. doi: 10.1128/jvi.35.3.603-618.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Beisel C., Hummel M., King W., Fennewald S., Cheung A., Heller M., Raab-Traub N., Kieff E. Epstein-Barr virus (B95-8) DNA VII: molecular cloning and detailed mapping. Proc Natl Acad Sci U S A. 1980 May;77(5):2999–3003. doi: 10.1073/pnas.77.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Edson C. M., Thorley-Lawson D. A. Epstein-Barr virus membrane antigens: characterization, distribution, and strain differences. J Virol. 1981 Jul;39(1):172–184. doi: 10.1128/jvi.39.1.172-184.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighny R. J., Farrell M. P., Pagano J. S. Polypeptide synthesis and phosphorylation in Epstein-Barr virus-infected cells. J Virol. 1980 May;34(2):455–463. doi: 10.1128/jvi.34.2.455-463.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighny R. J., Henry B. E., 2nd, Pagano J. S. Epstein-Barr virus polypeptides: effect of inhibition of viral DNA replication on their synthesis. J Virol. 1981 Jan;37(1):61–71. doi: 10.1128/jvi.37.1.61-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighny R. J., Henry B. E., 2nd, Pagano J. S. Epstein-Barr virus polypeptides: identification of early proteins and their synthesis and glycosylation. J Virol. 1981 Aug;39(2):651–655. doi: 10.1128/jvi.39.2.651-655.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P. Activation of Epstein-Barr virus by 5-bromodeoxyuridine in "virus-free" human cells (complement-fixing antigen-immunofluorescence-leukocytes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):83–85. doi: 10.1073/pnas.69.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given D., Kieff E. DNA of Epstein-Barr virus. IV. Linkage map of restriction enzyme fragments of the B95-8 and W91 strains of Epstein-Barr Virus. J Virol. 1978 Nov;28(2):524–542. doi: 10.1128/jvi.28.2.524-542.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given D., Kieff E. DNA of Epstein-Barr virus. VI. Mapping of the internal tandem reiteration. J Virol. 1979 Aug;31(2):315–324. doi: 10.1128/jvi.31.2.315-324.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given D., Yee D., Griem K., Kieff E. DNA of Epstein-Barr virus. V. Direct repeats of the ends of Epstein-Barr virus DNA. J Virol. 1979 Jun;30(3):852–862. doi: 10.1128/jvi.30.3.852-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Martos L. M., Walker J. L. Synthesis of Epstein-Barr virus after activation of the viral genome in a "virus-negative" human lymphoblastoid cell (Raji) made resistant to 5-bromodeoxyuridine (thymidine kinase-virus antigen-immunofluorescence-herpesvirus fingerprints). Proc Natl Acad Sci U S A. 1972 Jan;69(1):78–82. doi: 10.1073/pnas.69.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. D., Kieff E. DNA of Epstein-Barr virus. II. Comparison of the molecular weights of restriction endonuclease fragments of the DNA of Epstein-Barr virus strains and identification of end fragments of the B95-8 strain. J Virol. 1977 Aug;23(2):421–429. doi: 10.1128/jvi.23.2.421-429.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. D., Nogee L., Hayward G. S. Organization of repeated regions within the Epstein-Barr virus DNA molecule. J Virol. 1980 Jan;33(1):507–521. doi: 10.1128/jvi.33.1.507-521.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., Dambaugh T., Kieff E. Epstein-Barr virus DNA. IX. Variation among viral DNAs from producer and nonproducer infected cells. J Virol. 1981 May;38(2):632–648. doi: 10.1128/jvi.38.2.632-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallin B., Luka J., Klein G. Immunochemical characterization of Epstein-Barr virus-associated early and late antigens in n-butyrate-treated P3HR-1 cells. J Virol. 1979 Dec;32(3):710–716. doi: 10.1128/jvi.32.3.710-716.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi M., Sugawara K., Ito Y. Epstein-Barr virus-induced polypeptides: a comparative study with superinfected Raji, IUdR-Treated, and N-butyrate-treated P3HR-1 cells. Virology. 1981 Feb;109(1):72–81. doi: 10.1016/0042-6822(81)90472-4. [DOI] [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- King W., Thomas-Powell A. L., Raab-Traub N., Hawke M., Kieff E. Epstein-Barr virus RNA. V. Viral RNA in a restringently infected, growth-transformed cell line. J Virol. 1980 Nov;36(2):506–518. doi: 10.1128/jvi.36.2.506-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W., Van Santen V., Kieff E. Epstein-Barr virus RNA. VI. Viral RNA in restringently and abortively infected Raji cells. J Virol. 1981 May;38(2):649–660. doi: 10.1128/jvi.38.2.649-660.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner C. R., Sugden B. The structure of the termini of the DNA of Epstein-Barr virus. Cell. 1979 Jul;17(3):661–671. doi: 10.1016/0092-8674(79)90273-3. [DOI] [PubMed] [Google Scholar]
- Klein G., Pearson G., Nadkarni J. S., Nadkarni J. J., Klein E., Henle G., Henle W., Clifford P. Relation between Epstein-Barr viral and cell membrane immunofluorescence of Burkitt tumor cells. I. Dependence of cell membrane immunofluorescence on presence of EB virus. J Exp Med. 1968 Nov 1;128(5):1011–1020. doi: 10.1084/jem.128.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Andrews N. C., Miller G., Steitz J. A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuller-Lantzsch N., Georg B., Yamamoto N., zur Hausen H. Epstein-Barr virus-induced proteins. II. Analysis of surface polypeptides from EBV-producing and -superinfected cells by immunoprecipitation. Virology. 1980 Apr 30;102(2):401–411. doi: 10.1016/0042-6822(80)90107-5. [DOI] [PubMed] [Google Scholar]
- Miller G., Coope D., Niederman J., Pagano J. Biological properties and viral surface antigens of Burkitt lymphoma- and mononucleosis- derived strains of Epstein-Barr virus released from transformed marmoset cells. J Virol. 1976 Jun;18(3):1071–1080. doi: 10.1128/jvi.18.3.1071-1080.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Georg B., Yamamoto N., zur Hausen H. Epstein-Barr virus-induced proteins. III. Analysis of polypeptides from P3HR-1-EBV-superinfected NC37 cells by immunoprecipitation. Virology. 1980 Apr 15;102(1):231–233. doi: 10.1016/0042-6822(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Yamamoto N., zur Hausen H. Analysis of early and late Epstein-Barr virus associated polypeptides by immunoprecipitation. Virology. 1979 Sep;97(2):378–387. doi: 10.1016/0042-6822(79)90348-9. [DOI] [PubMed] [Google Scholar]
- Orellana T., Kieff E. Epstein-barr virus-specific RNA. II. Analysis of polyadenylated viral RNA in restringent, abortive, and prooductive infections. J Virol. 1977 May;22(2):321–330. doi: 10.1128/jvi.22.2.321-330.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell A. L., King W., Kieff E. Epstein-Barr virus-specific RNA. III. Mapping of DNA encoding viral RNA in restringent infection. J Virol. 1979 Jan;29(1):261–274. doi: 10.1128/jvi.29.1.261-274.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett R. F., Hayward S. D., Kieff E. D. DNA of Epstein-Barr virus. I. Comparative studies of the DNA of Epstein-Barr virus from HR-1 and B95-8 cells: size, structure, and relatedness. J Virol. 1975 Mar;15(3):556–559. doi: 10.1128/jvi.15.3.556-559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Traub N., Dambaugh T., Kieff E. DNA of Epstein-Barr virus VIII: B95-8, the previous prototype, is an unusual deletion derivative. Cell. 1980 Nov;22(1 Pt 1):257–267. doi: 10.1016/0092-8674(80)90173-7. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N., Pritchett R., Kieff E. DNA of Epstein-Barr virus. III. Identification of restriction enzyme fragments that contain DNA sequences which differ among strains of Epstein-Barr virus. J Virol. 1978 Aug;27(2):388–398. doi: 10.1128/jvi.27.2.388-398.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosa M. D., Gottlieb E., Lerner M. R., Steitz J. A. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol. 1981 Sep;1(9):785–796. doi: 10.1128/mcb.1.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Manley J., Fire A., Gefter M. Regulation of adenovirus mRNA synthesis. Ann N Y Acad Sci. 1980;354:1–15. doi: 10.1111/j.1749-6632.1980.tb27954.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Neubauer R. H., Rabin H., Mazur R. A. Correlation between Epstein-Barr virus membrane antigen and three large cell surface glycoproteins. J Virol. 1979 Dec;32(3):885–894. doi: 10.1128/jvi.32.3.885-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Klein G. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol. 1976 Apr;18(1):151–155. doi: 10.1128/jvi.18.1.151-155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., zur Hausen H. Effect of inhibition of DNA synthesis on Epstein--Barr virus induction by tumor promoters. Virology. 1980 Feb;101(1):104–110. doi: 10.1016/0042-6822(80)90487-0. [DOI] [PubMed] [Google Scholar]
- van Santen V., Cheung A., Kieff E. Epstein-Barr virus RNA VII: size and direction of transcription of virus-specified cytoplasmic RNAs in a transformed cell line. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1930–1934. doi: 10.1073/pnas.78.3.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]