Abstract
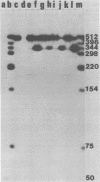
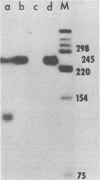
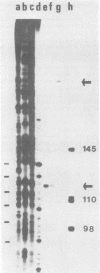
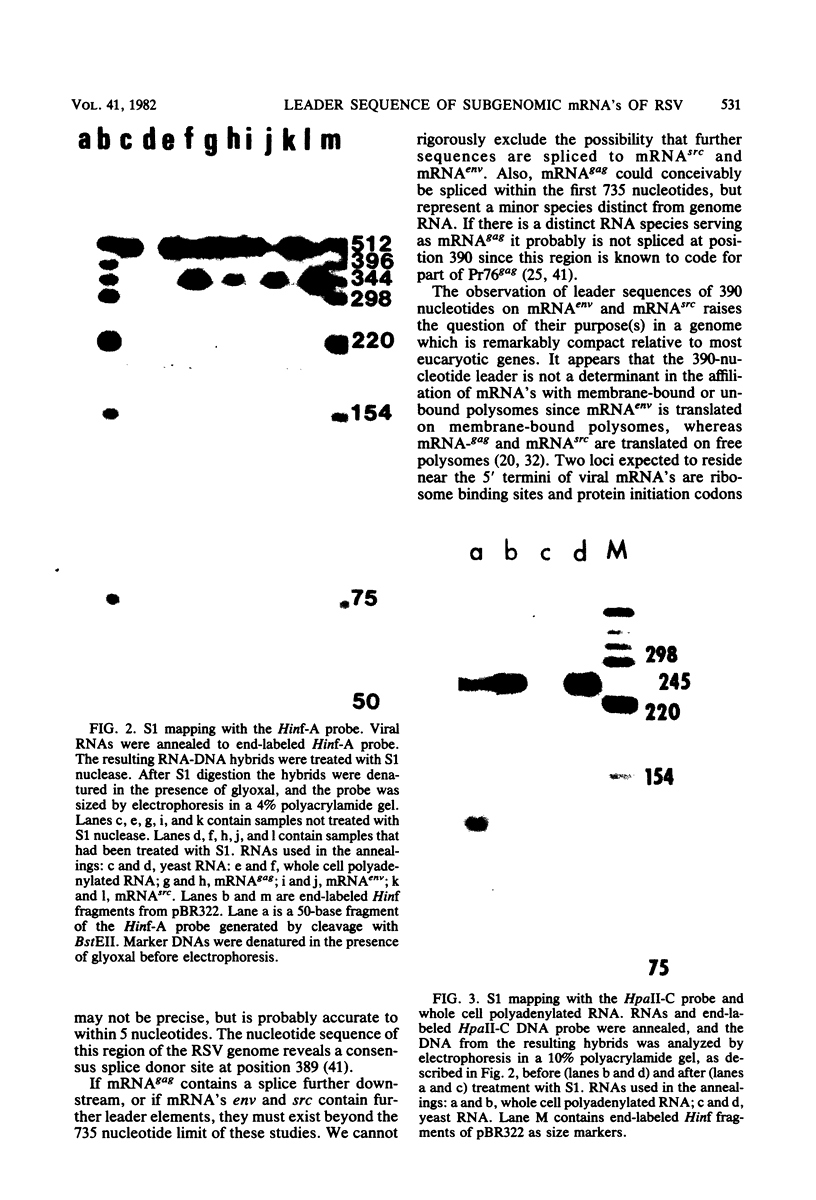
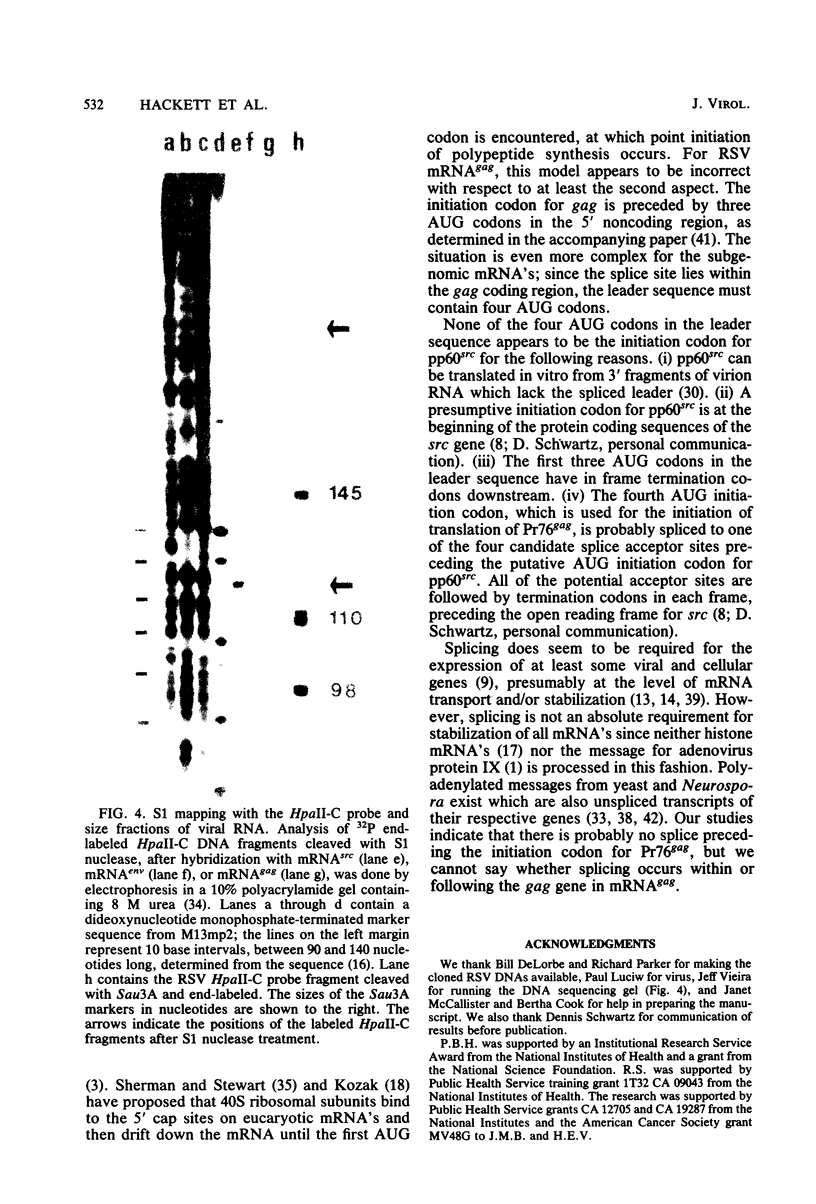
The subgenomic mRNA's of Rous sarcoma virus share a common 5' leader sequence spliced from genomic RNA. We have examined the 5' terminal sequences of four Rous sarcoma virus RNAs: virion RNA and three species of intracellular mRNA which direct the synthesis of the RSV gene products. The lengths of the leaders on the RNAs were determined by the extent that they could protect cloned Rous sarcoma virus DNA fragments from S1 nuclease digestion after RNA-DNA hybridization. We found that the subgenomic mRNA's that direct the synthesis of the env and src gene products have uninterrupted spliced leader sequences of approximately 390 nucleotides, whereas virion RNA and full-length intracellular viral RNA have 5' termini homologous to the cloned viral DNA probe over at least the first 735 bases. In the accompanying manuscript we have determined the nucleotide sequence of the 5' end of the Rous sarcoma virus genome, including the candidate splice donor site identified here (Swanstrom et al., J. Virol. 41:535-541, 1982).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleström P., Akusjärvi G., Perricaudet M., Mathews M. B., Klessig D. F., Pettersson U. The gene for polypeptide IX of adenovirus type 2 and its unspliced messenger RNA. Cell. 1980 Mar;19(3):671–681. doi: 10.1016/s0092-8674(80)80044-4. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Brugge J., Erikson E., Collett M. S., Erikson R. I. Peptide analysis of the transformation-specific antigen from avian sarcoma virus-transformed cells. J Virol. 1978 Jun;26(3):773–782. doi: 10.1128/jvi.26.3.773-782.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Weiss S. R., Varmus H. E., Bishop J. M. At least 104 nucleotides are transposed from the 5' terminus of the avian sarcoma virus genome to the 5' termini of smaller viral mRNAs. Cell. 1978 Sep;15(1):79–91. doi: 10.1016/0092-8674(78)90084-3. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. M. Nucleotide sequence of an avian sarcoma virus oncogene (src) and proposed amino acid sequence for gene product. Nature. 1980 Sep 18;287(5779):198–203. doi: 10.1038/287198a0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Implications of RNA-RNA splicing in evolution of eukaryotic cells. Science. 1978 Dec 22;202(4374):1257–1260. doi: 10.1126/science.364651. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Roeder R. G. Definition of a novel promoter for the major adenovirus-associated virus mRNA. Cell. 1980 Nov;22(1 Pt 1):231–242. doi: 10.1016/0092-8674(80)90171-3. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Identification of regulatory sequences in the prelude sequences of an H2A histone gene by the study of specific deletion mutants in vivo. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1432–1436. doi: 10.1073/pnas.77.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Lai C. J., Dhar R., Khoury G. Splicing as a requirement for biogenesis of functional 16S mRNA of simian virus 40. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4317–4321. doi: 10.1073/pnas.76.9.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Varmus H. E., Bishop J. M. The genesis of Rous sarcoma virus messenger RNAs. Virology. 1981 Jul 30;112(2):714–728. doi: 10.1016/0042-6822(81)90316-0. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Splicing and the formation of stable RNA. Cell. 1979 Dec;18(4):1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Kedes L. H. Histone genes and histone messengers. Annu Rev Biochem. 1979;48:837–870. doi: 10.1146/annurev.bi.48.070179.004201. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Collett M. S., Lau A. F., Perdue M. L., Leis J. P., Faras A. J. Evidence for splicing of avian sarcoma virus 5'-terminal genomic sequences into viral-specific RNA in infected cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1284–1288. doi: 10.1073/pnas.75.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Varmus H. E., Bishop J. M. Virus-specific messenger RNAs in permissive cells infected by avian sarcoma virus. J Biol Chem. 1979 Aug 25;254(16):8015–8022. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Vogt V. M., Ripley S., Eisenman R. N. The NH2-terminal sequence of the avian oncovirus gag precursor polyprotein (Pr76gag). Virology. 1978 Dec;91(2):423–433. doi: 10.1016/0042-6822(78)90388-4. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Marciani D. J., Papas T. S. Cell-free synthesis of the precursor polypeptide for avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4951–4954. doi: 10.1073/pnas.74.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Harvey R., Smith A. E. The size of Rous sarcoma virus mRNAs active in cell-free translation. Nature. 1977 Aug 4;268(5619):416–420. doi: 10.1038/268416a0. [DOI] [PubMed] [Google Scholar]
- Pawson T., Martin G. S., Smith A. E. Cell-free translation of virion RNA from nondefective and transformation-defective Rous sarcoma viruses. J Virol. 1976 Sep;19(3):950–967. doi: 10.1128/jvi.19.3.950-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Mellon P., Duesberg P. H., Martin G. S. env Gene of Rous sarcoma virus: identification of the gene product by cell-free translation. J Virol. 1980 Mar;33(3):993–1003. doi: 10.1128/jvi.33.3.993-1003.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Jovanovich S., Erikson R. L. Sites of synthesis of viral proteins in avian sarcoma virus-infected chicken cells. J Virol. 1980 Sep;35(3):629–636. doi: 10.1128/jvi.35.3.629-636.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzkin B., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):487–491. doi: 10.1073/pnas.74.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G., Hanafusa H. Microinjection analysis of envelope-glycoprotein messenger activities of avian leukosis viral RNAs. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1614–1618. doi: 10.1073/pnas.74.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Kuhnert L. K. Evidence for the identity of shared 5'-terminal sequences between genome RNA and subgenomic mRNA's of B77 avian sarcoma virus. J Virol. 1979 Nov;32(2):536–545. doi: 10.1128/jvi.32.2.536-545.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Davis R. W. Production of a functional eukaryotic enzyme in Escherichia coli: cloning and expression of the yeast structural gene for imidazole-glycerolphosphate dehydratase (his3). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5255–5259. doi: 10.1073/pnas.74.12.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K. N. Segments of simian virus 40 DNA spanning most of the leader sequence of the major late viral messenger RNA are dispensable. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2556–2560. doi: 10.1073/pnas.76.6.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Varmus H. E., Bishop J. M. Nucleotide sequence of the 5' noncoding region and part of the gag gene of Rous sarcoma virus. J Virol. 1982 Feb;41(2):535–541. doi: 10.1128/jvi.41.2.535-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Hautala J. A., Jacobson J. W., Giles N. H., Kushner S. R. Expression in Escherichia coli K-12 of the structural gene for catabolic dehydroquinase of Neurospora crassa. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3508–3512. doi: 10.1073/pnas.74.8.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Ortiz S. Retroviruses as mutagens: insertion and excision of a nontransforming provirus alter expression of a resident transforming provirus. Cell. 1981 Jul;25(1):23–36. doi: 10.1016/0092-8674(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Hackett P. B., Oppermann H., Ullrich A., Levintow L., Bishop J. M. Cell-free translation of avian sarcoma virus RNA: suppression of the gag termination codon does not augment synthesis of the joint gag/pol product. Cell. 1978 Oct;15(2):607–614. doi: 10.1016/0092-8674(78)90029-6. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- von der Helm K., Duesberg P. H. Translation of Rous sarcoma virus RNA in a cell-free system from ascites Krebs II cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):614–618. doi: 10.1073/pnas.72.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]