Abstract
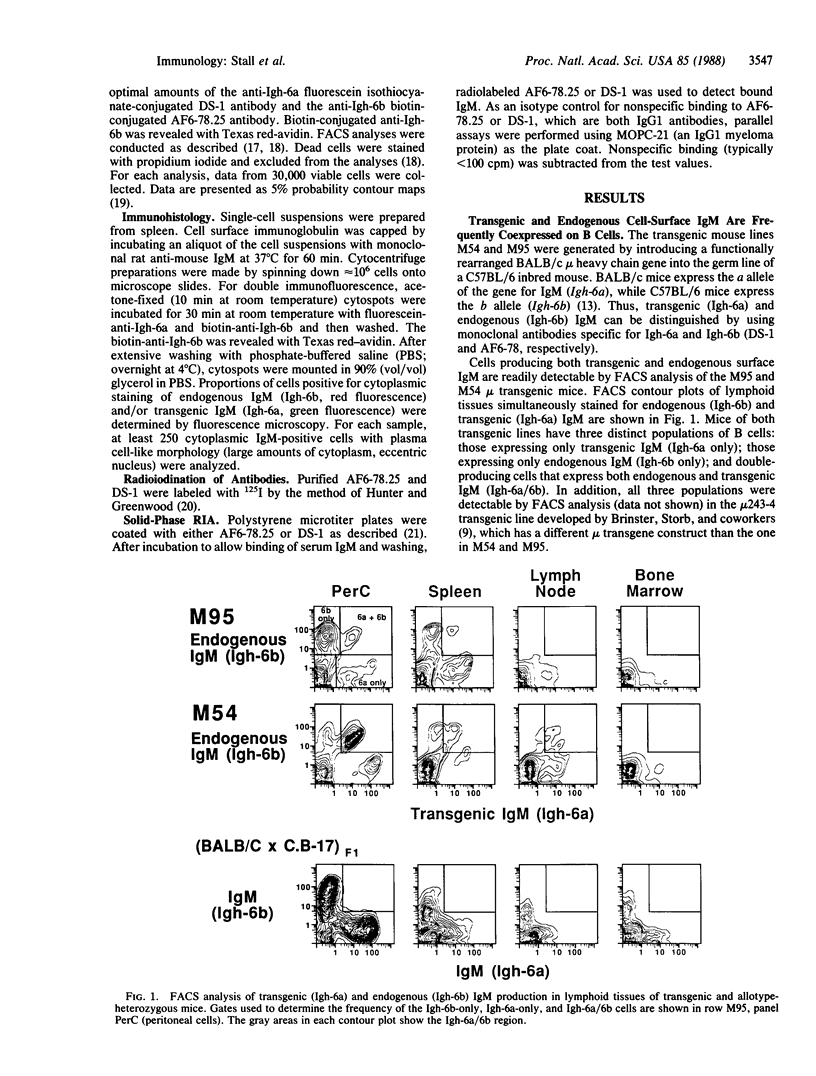
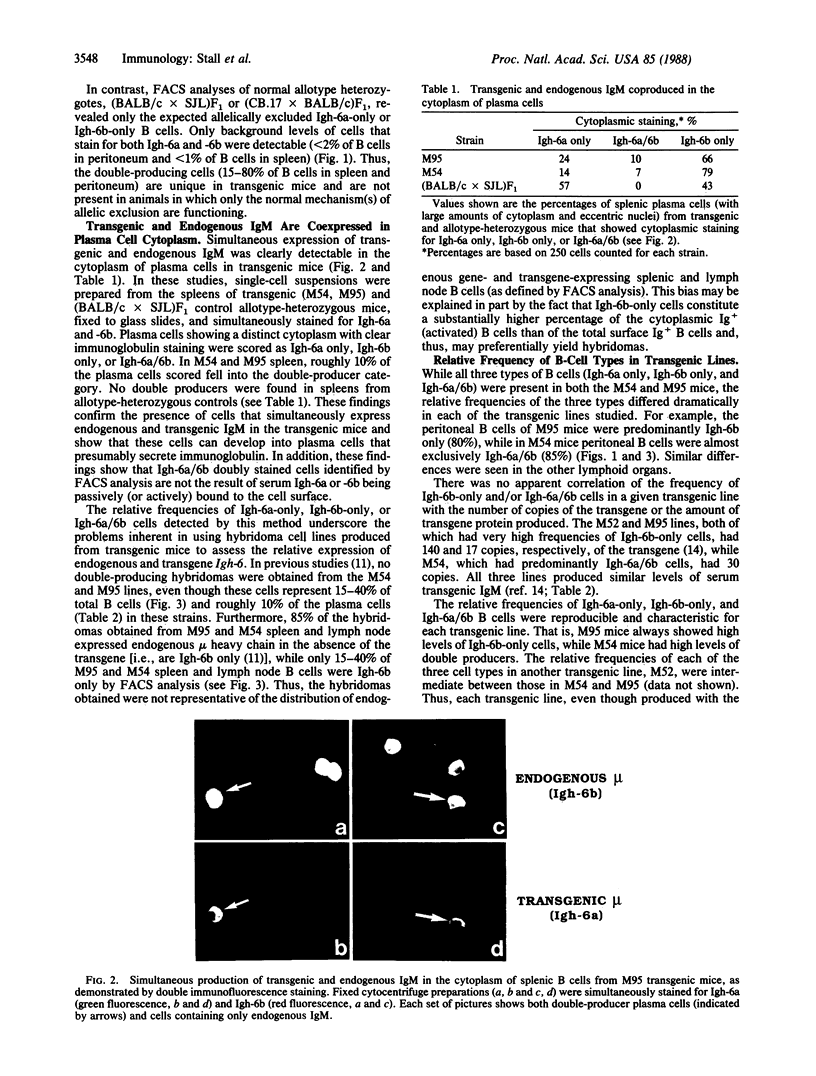
Transgenic mice carrying immunoglobulin genes coding for mu heavy chain and kappa light chain have been used to study the mechanisms involved in allelic and isotypic exclusion. We report here that individual cells from transgenic mice carrying a functionally rearranged mu heavy chain gene (capable of generating both membrane and secreted forms of IgM) can rearrange an endogenous mu heavy chain gene and simultaneously produce both transgenic and endogenous IgM. These "double-producing" cells express both endogenous and transgenic IgM in the cytoplasm (detected by immunohistology) and on the cell surface (detected by multiparameter fluorescence-activated cell sorter analysis). In addition, they secrete mixed IgM molecules containing both transgenic and endogenous mu heavy chains (detected in serum by radioimmune assay). The transgenic mice studied also have relatively large numbers of cells that produce endogenous immunoglobulin in the absence of detectable transgenic immunoglobulin ("endogenous-only cells"). The mechanisms that generate double-producing cells and endogenous-only cells appear to be under genetic control because the frequencies of these B-cell populations are characteristic for a given transgenic line. Thus, our findings indicate that more is involved in triggering allelic exclusion than the simple presence or absence of membrane mu heavy chains (as has been previously postulated).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Herzenberg L. A., Black S. J., Loken M. R., Okumura K., van der Loo W., Osborne B. A., Hewgill D., Goding J. W., Gutman G. Surface markers and functional relationships of cells involved in murine B-lymphocyte differentiation. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):33–45. doi: 10.1101/sqb.1977.041.01.007. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Braun J., Weaver D., Baltimore D., Herzenberg L. A., Grosschedl R. Depletion of the predominant B-cell population in immunoglobulin mu heavy-chain transgenic mice. Nature. 1987 Sep 3;329(6134):71–73. doi: 10.1038/329071a0. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Iglesias A., Lamers M., Köhler G. Expression of immunoglobulin delta chain causes allelic exclusion in transgenic mice. Nature. 1987 Dec 3;330(6147):482–484. doi: 10.1038/330482a0. [DOI] [PubMed] [Google Scholar]
- Jones P. P., Tacier-Eugster H., Herzenberg L. A. Lymphocyte commitment to Ig allotype and class. Ann Immunol (Paris) 1974 Jan;125C(1-2):271–276. [PubMed] [Google Scholar]
- Nussenzweig M. C., Shaw A. C., Sinn E., Danner D. B., Holmes K. L., Morse H. C., 3rd, Leder P. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987 May 15;236(4803):816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- Pernis B., Chiappino G., Kelus A. S., Gell P. G. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965 Nov 1;122(5):853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi S., Köhler G. Transmission and expression of a specific pair of rearranged immunoglobulin mu and kappa genes in a transgenic mouse line. 1985 Mar 28-Apr 3Nature. 314(6009):330–334. doi: 10.1038/314330a0. [DOI] [PubMed] [Google Scholar]
- Stall A. M., Loken M. R. Allotypic specificities of murine IgD and IgM recognized by monoclonal antibodies. J Immunol. 1984 Feb;132(2):787–795. [PubMed] [Google Scholar]
- Storb U., Pinkert C., Arp B., Engler P., Gollahon K., Manz J., Brady W., Brinster R. L. Transgenic mice with mu and kappa genes encoding antiphosphorylcholine antibodies. J Exp Med. 1986 Aug 1;164(2):627–641. doi: 10.1084/jem.164.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Weaver D., Costantini F., Imanishi-Kari T., Baltimore D. A transgenic immunoglobulin mu gene prevents rearrangement of endogenous genes. Cell. 1985 Aug;42(1):117–127. doi: 10.1016/s0092-8674(85)80107-0. [DOI] [PubMed] [Google Scholar]
- Weaver D., Reis M. H., Albanese C., Costantini F., Baltimore D., Imanishi-Kari T. Altered repertoire of endogenous immunoglobulin gene expression in transgenic mice containing a rearranged mu heavy chain gene. Cell. 1986 Apr 25;45(2):247–259. doi: 10.1016/0092-8674(86)90389-2. [DOI] [PubMed] [Google Scholar]