Abstract
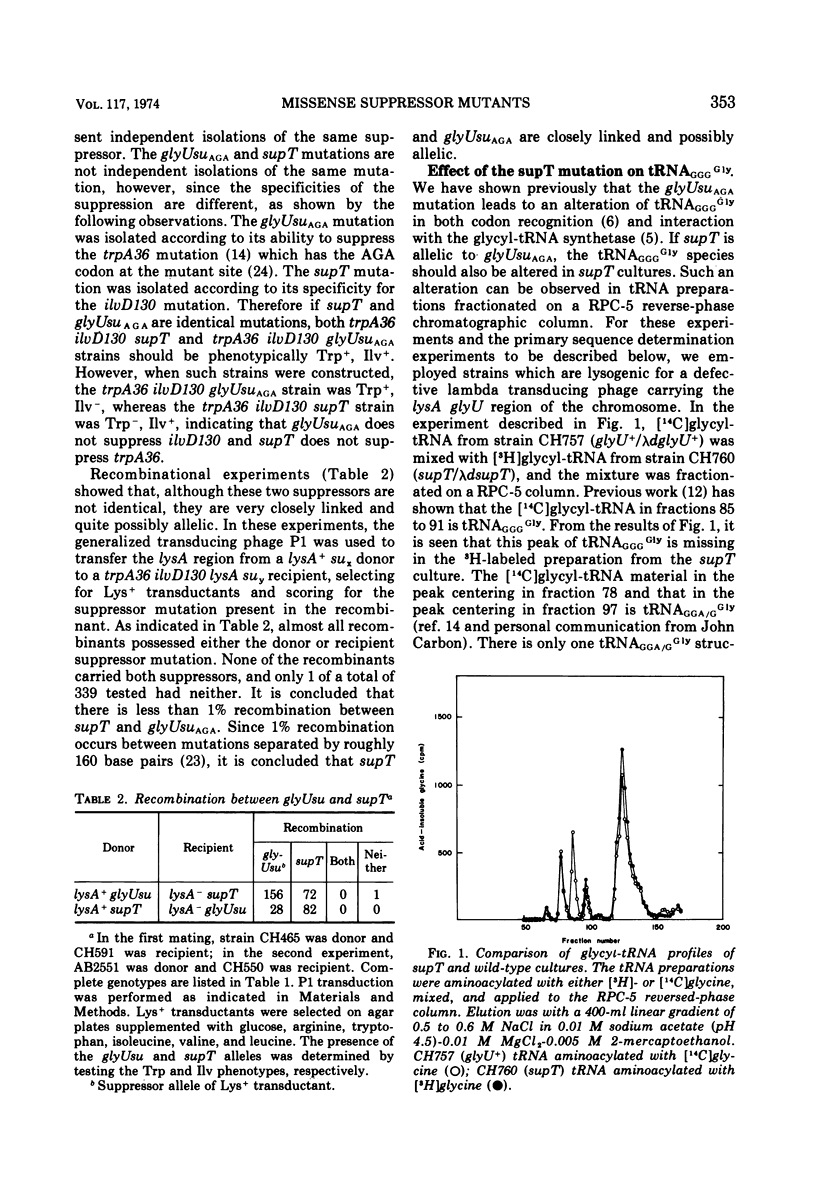
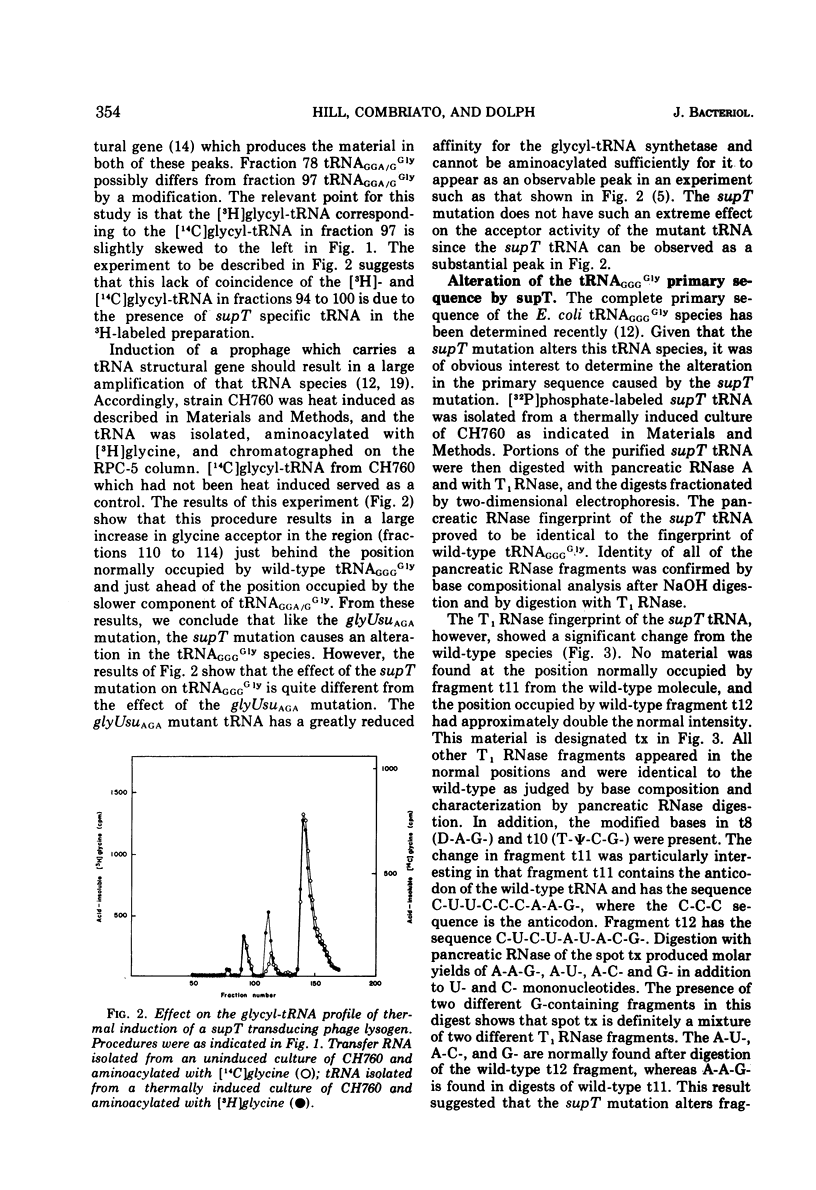
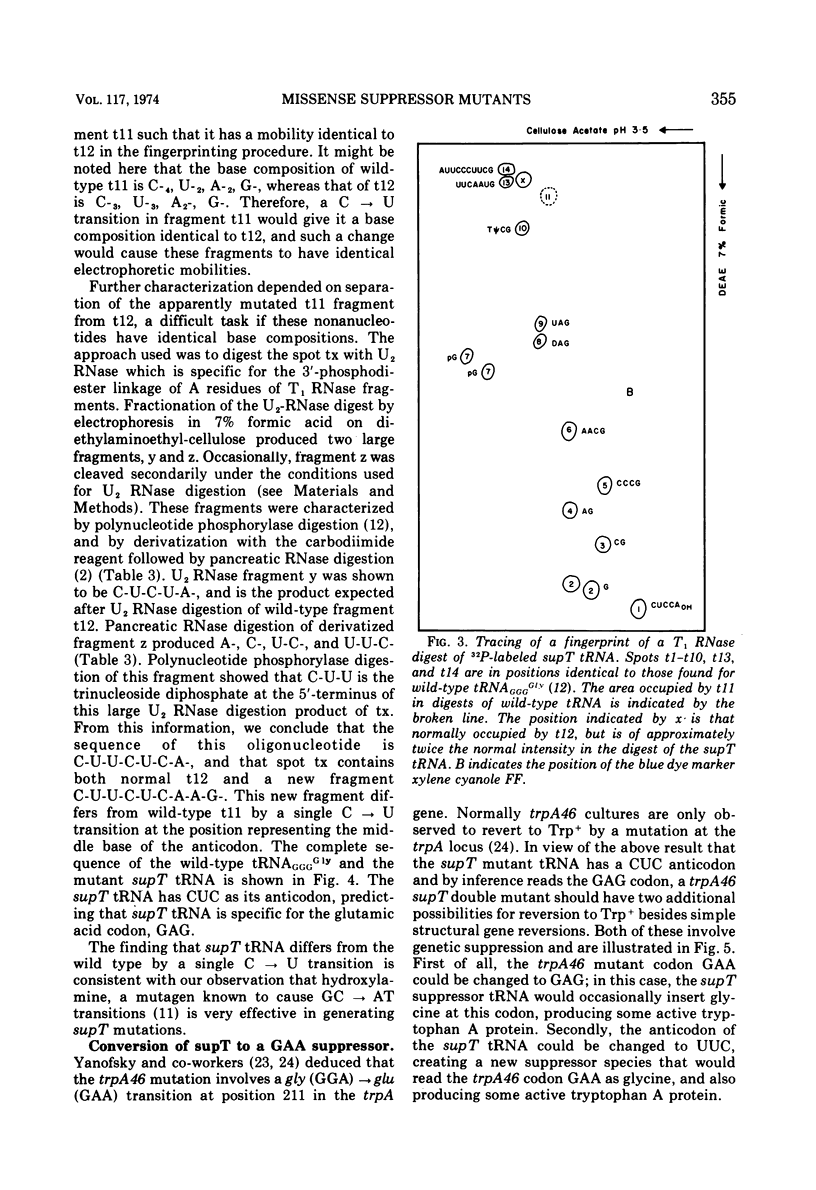
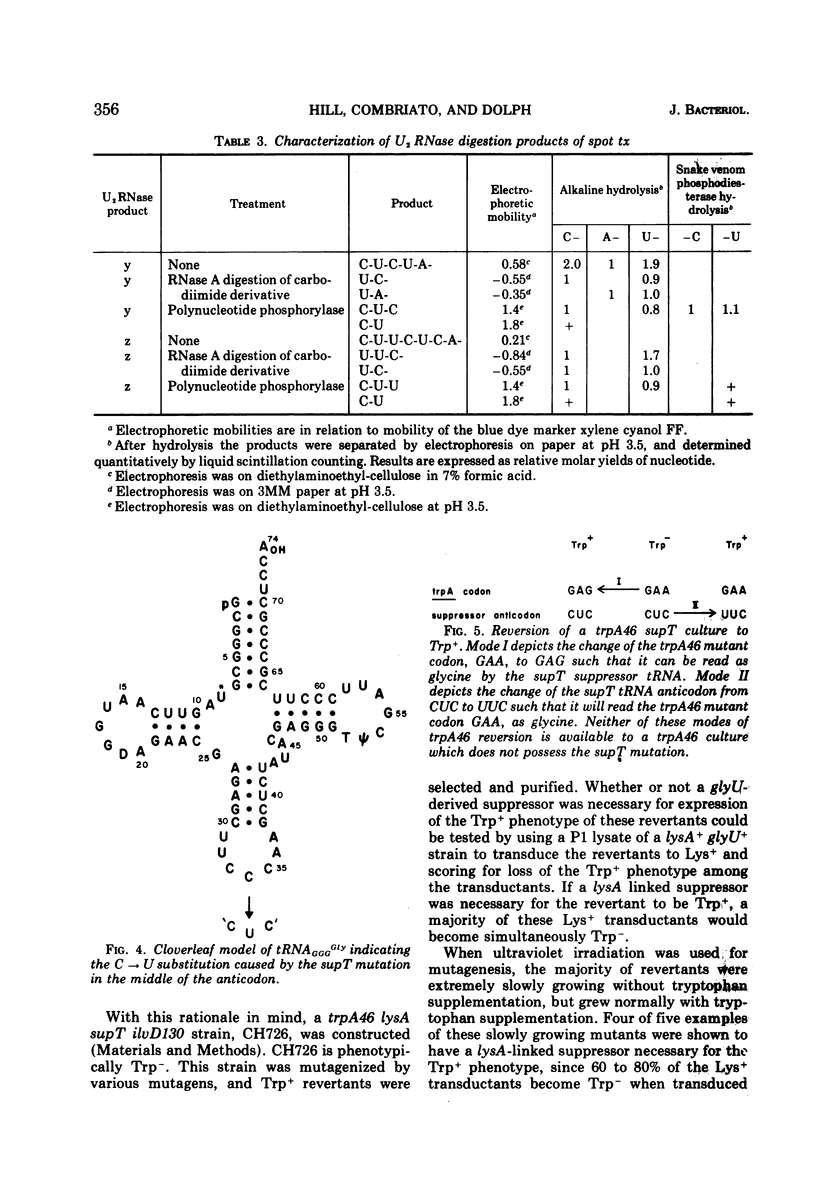
The Escherichia coli suppressor mutation, supT, has been shown to cause a C → U substitution in the middle position of the tRNAGGGGly anticodon. This is the same tRNA species that is altered by the glyUsuAGA mutation studied previously. This finding indicates that the supT mutant tRNA reads the glutamic acid codon, GAG. The supT suppressor has also been converted to a new suppressor, called glyUsuGAA, which will suppress the GAA mutation, trpA46. The in vivo suppression efficiencies of each of these three missense suppressors has been measured and are as follows: glyUsuAGA, 3.6%; supT, 1.6%; and glyUsuGAA, 0.4%. Mistranslation by these mutant glycine tRNA species has no adverse affects on cell growth since cultures possessing the suppressors grow as fast as cells without. The supT tRNA species can be observed as a peak in the profile of glycyl-tRNA fractionated on a RPC-5 chromatographic column, indicating that the mutant tRNA can be aminoacylated with reasonable efficiency. This finding contrasts with previous findings concerning the glyUsuAGA mutant tRNA which is not significantly aminoacylated under the same conditions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. The effect of tRNA concentration on the rate of protein synthesis. Proc Natl Acad Sci U S A. 1969 Feb;62(2):566–573. doi: 10.1073/pnas.62.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODY S., YANOFSKY C. Suppressor gene alteration of protein primary structure. Proc Natl Acad Sci U S A. 1963 Jul;50:9–16. doi: 10.1073/pnas.50.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Location of the structural gene for glycyl ribonucleic acid synthetase by means of a strain of Escherichia coli possessing an unusual enzyme. Z Vererbungsl. 1966;98(3):187–192. doi: 10.1007/BF00888946. [DOI] [PubMed] [Google Scholar]
- Carbon J., Squires C., Hill C. W. Genetically altered tRNA-Gly subspecies in E. coli. Cold Spring Harb Symp Quant Biol. 1969;34:505–512. doi: 10.1101/sqb.1969.034.01.057. [DOI] [PubMed] [Google Scholar]
- Carbon J., Squires C., Hill C. W. Glycine transfer RNA of Escherichia coli. II. Impaired GGA-recognition in strains containing a genetically altered transfer RNA; reversal by a secondary suppressor mutation. J Mol Biol. 1970 Sep 28;52(3):571–584. doi: 10.1016/0022-2836(70)90420-1. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggertsson G., Adelberg E. A. Map positions and specificities of suppressor mutations in Escherichia coli K-12. Genetics. 1965 Aug;52(2):319–340. doi: 10.1093/genetics/52.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESE E., BAUTZ E., FREESE E. B. The chemical and mutagenic specificity of hydroxylamine. Proc Natl Acad Sci U S A. 1961 Jun 15;47:845–855. doi: 10.1073/pnas.47.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Combriato G., Steinhart W., Riddle D. L., Carbon J. The nucleotide sequence of the GGG-specific glycine transfer ribonucleic acid of Escherichia coli and of Salmonella typhimurium. J Biol Chem. 1973 Jun 25;248(12):4252–4262. [PubMed] [Google Scholar]
- Hill C. W., Foulds J., Soll L., Berg P. Instability of a missense suppressor resulting from a duplication of genetic material. J Mol Biol. 1969 Feb 14;39(3):563–581. doi: 10.1016/0022-2836(69)90146-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Ostrem D. L., Berg P. Glycyl-tRNA synthetase: an oligomeric protein containing dissimilar subunits. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1967–1974. doi: 10.1073/pnas.67.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle D. L., Roth J. R. Frameshift suppressors. 3. Effects of suppressor mutations on transfer RNA. J Mol Biol. 1972 May 28;66(3):495–506. doi: 10.1016/0022-2836(72)90429-9. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Squires C., Carbon J., Hill C. W. Glycine transfer RNA of Escherichia coli. I. Structural genes for two glycine tRNA species. J Mol Biol. 1970 Sep 28;52(3):557–569. doi: 10.1016/0022-2836(70)90419-5. [DOI] [PubMed] [Google Scholar]
- Squires C., Konrad B., Kirschbaum J., Carbon J. Three adjacent transfer RNA genes in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):438–441. doi: 10.1073/pnas.70.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yanofsky C., Horn V. Tryptophan synthetase chain positions affected by mutations near the ends of the genetic map of trpA of Escherichia coli. J Biol Chem. 1972 Jul 25;247(14):4494–4498. [PubMed] [Google Scholar]
- Yanofsky C., Ito J., Horn V. Amino acid replacements and the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:151–162. doi: 10.1101/sqb.1966.031.01.023. [DOI] [PubMed] [Google Scholar]


