Abstract
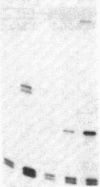
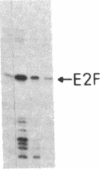
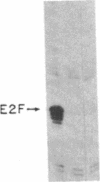
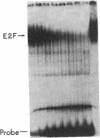
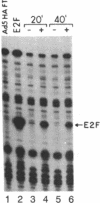
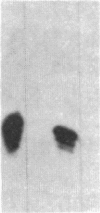
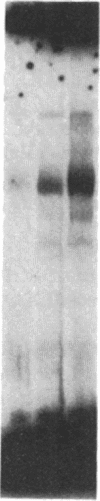
Adenovirus infection induces a large increase in the DNA binding activity of a cellular transcription factor that is utilized by the viral E2 promoter and termed E2F. Using cell-free extracts, we have developed an assay for the in vitro activation of DNA binding activity of E2F. E2F activity is undetectable in HeLa extracts but upon incubation with a fraction from adenovirus-infected cells, there is an ATP-dependent increase in E2F DNA binding activity. This increase does not occur using an equivalent fraction from dl312 (E1A-)-infected cells. Incubation of E2F with phosphatase inactivates E2F binding activity. Incubation of the phosphatase-inactivated E2F with an infected cell fraction restores E2F activity as does incubation with a known protein kinase. In contrast, incubation with an extract from mock-infected cells does not restore activity. We conclude that the DNA binding activity of E2F is regulated by phosphorylation in an E1A-dependent manner.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger S. L., Folk W. R. Differential activation of RNA polymerase III-transcribed genes by the polyomavirus enhancer and the adenovirus E1A gene products. Nucleic Acids Res. 1985 Feb 25;13(4):1413–1428. doi: 10.1093/nar/13.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkaim R., Goding C., Kédinger C. The adenovirus-2 EIIa early gene promoter: sequences required for efficient in vitro and in vivo transcription. Nucleic Acids Res. 1983 Oct 25;11(20):7105–7117. doi: 10.1093/nar/11.20.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B., Krippl B., Andrisani O., Jones N., Westphal H., Rosenberg M. E1A 13S and 12S mRNA products made in Escherichia coli both function as nucleus-localized transcription activators but do not directly bind DNA. Mol Cell Biol. 1985 Oct;5(10):2653–2661. doi: 10.1128/mcb.5.10.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R. B., Berk A. J. Cis-acting induction of adenovirus transcription. Cell. 1983 Jul;33(3):683–693. doi: 10.1016/0092-8674(83)90011-9. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Feldman L. T., Berk A. J. Transcription of class III genes activated by viral immediate early proteins. Science. 1985 Oct 25;230(4724):447–450. doi: 10.1126/science.2996135. [DOI] [PubMed] [Google Scholar]
- Goding C., Jalinot P., Zajchowski D., Boeuf H., Kédinger C. Sequence-specific trans-activation of the adenovirus EIIa early promoter by the viral EIV transcription unit. EMBO J. 1985 Jun;4(6):1523–1528. doi: 10.1002/j.1460-2075.1985.tb03812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hoeffler W. K., Kovelman R., Roeder R. G. Activation of transcription factor IIIC by the adenovirus E1A protein. Cell. 1988 Jun 17;53(6):907–920. doi: 10.1016/s0092-8674(88)90409-6. [DOI] [PubMed] [Google Scholar]
- Hoeffler W. K., Roeder R. G. Enhancement of RNA polymerase III transcription by the E1A gene product of adenovirus. Cell. 1985 Jul;41(3):955–963. doi: 10.1016/s0092-8674(85)80076-3. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Feldman L. T., Nevins J. R. Activation of gene expression by adenovirus and herpesvirus regulatory genes acting in trans and by a cis-acting adenovirus enhancer element. Cell. 1983 Nov;35(1):127–136. doi: 10.1016/0092-8674(83)90215-5. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Hart R. P., Nevins J. R. An enhancer-like element in the adenovirus E2 promoter contains sequences essential for uninduced and E1A-induced transcription. Proc Natl Acad Sci U S A. 1985 Jan;82(2):381–385. doi: 10.1073/pnas.82.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale M. J., Kao H. T., Feldman L. T., Nevins J. R., Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale M. J., Nevins J. R. Adenovirus 5 E2 transcription unit: an E1A-inducible promoter with an essential element that functions independently of position or orientation. Mol Cell Biol. 1984 May;4(5):875–882. doi: 10.1128/mcb.4.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalinot P., Devaux B., Kédinger C. The abundance and in vitro DNA binding of three cellular proteins interacting with the adenovirus EIIa early promoter are not modified by the EIa gene products. Mol Cell Biol. 1987 Oct;7(10):3806–3817. doi: 10.1128/mcb.7.10.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R. E., Kaufman R. J., Sharp P. A. Regulation of transcription of the adenovirus EII promoter by EIa gene products: absence of sequence specificity. Mol Cell Biol. 1984 Oct;4(10):1970–1977. doi: 10.1128/mcb.4.10.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. E1A transcription induction: enhanced binding of a factor to upstream promoter sequences. Science. 1986 Feb 14;231(4739):719–722. doi: 10.1126/science.2935935. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Role of an adenovirus E2 promoter binding factor in E1A-mediated coordinate gene control. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2180–2184. doi: 10.1073/pnas.84.8.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Hai T. Y., SivaRaman L., Thimmappaya B., Hurst H. C., Jones N. C., Green M. R. A cellular protein, activating transcription factor, activates transcription of multiple E1A-inducible adenovirus early promoters. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8355–8359. doi: 10.1073/pnas.84.23.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C., Courtois G., Eng C., Berk A. Complete transformation by adenovirus 2 requires both E1A proteins. Cell. 1984 Apr;36(4):951–961. doi: 10.1016/0092-8674(84)90045-x. [DOI] [PubMed] [Google Scholar]
- Murthy S. C., Bhat G. P., Thimmappaya B. Adenovirus EIIA early promoter: transcriptional control elements and induction by the viral pre-early EIA gene, which appears to be sequence independent. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2230–2234. doi: 10.1073/pnas.82.8.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Definition and mapping of adenovirus 2 nuclear transcription. Methods Enzymol. 1980;65(1):768–785. doi: 10.1016/s0076-6879(80)65072-1. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Ginsberg H. S., Blanchard J. M., Wilson M. C., Darnell J. E., Jr Regulation of the primary expression of the early adenovirus transcription units. J Virol. 1979 Dec;32(3):727–733. doi: 10.1128/jvi.32.3.727-733.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Regulation of early adenovirus gene expression. Microbiol Rev. 1987 Dec;51(4):419–430. doi: 10.1128/mr.51.4.419-430.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri P., Rooney R., Nevins J. R. Identification of an E1A-inducible cellular factor that interacts with regulatory sequences within the adenovirus E4 promoter. EMBO J. 1987 Dec 20;6(13):4073–4081. doi: 10.1002/j.1460-2075.1987.tb02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel R., Kovesdi I., Nevins J. R. Activation of a preexisting cellular factor as a basis for adenovirus E1A-mediated transcription control. Proc Natl Acad Sci U S A. 1988 Jan;85(2):387–390. doi: 10.1073/pnas.85.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel R., Kovesdi I., Nevins J. R. Developmental control of a promoter-specific factor that is also regulated by the E1A gene product. Cell. 1987 Feb 13;48(3):501–506. doi: 10.1016/0092-8674(87)90200-5. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Jones R. L., Cepko C. L., Sharp P. A., Roberts B. E. Expression of early adenovirus genes requires a viral encoded acidic polypeptide. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6121–6125. doi: 10.1073/pnas.78.10.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Fisch T. M., Benecke B. J., Nevins J. R., Heintz N. Definition of multiple, functionally distinct TATA elements, one of which is a target in the hsp70 promoter for E1A regulation. Cell. 1988 Mar 11;52(5):723–729. doi: 10.1016/0092-8674(88)90410-2. [DOI] [PubMed] [Google Scholar]
- SivaRaman L., Subramanian S., Thimmappaya B. Identification of a factor in HeLa cells specific for an upstream transcriptional control sequence of an EIA-inducible adenovirus promoter and its relative abundance in infected and uninfected cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5914–5918. doi: 10.1073/pnas.83.16.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Shenk T. Dissection of overlapping functions within the adenovirus type 5 E1A gene. EMBO J. 1984 Aug;3(8):1907–1912. doi: 10.1002/j.1460-2075.1984.tb02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Rosser D. S., Schmidt M. C., Berk A. A TATA box implicated in E1A transcriptional activation of a simple adenovirus 2 promoter. Nature. 1987 Apr 2;326(6112):512–515. doi: 10.1038/326512a0. [DOI] [PubMed] [Google Scholar]
- Yee A. S., Raychaudhuri P., Jakoi L., Nevins J. R. The adenovirus-inducible factor E2F stimulates transcription after specific DNA binding. Mol Cell Biol. 1989 Feb;9(2):578–585. doi: 10.1128/mcb.9.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee A. S., Reichel R., Kovesdi I., Nevins J. R. Promoter interaction of the E1A-inducible factor E2F and its potential role in the formation of a multi-component complex. EMBO J. 1987 Jul;6(7):2061–2068. doi: 10.1002/j.1460-2075.1987.tb02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga S., Dean N., Han M., Berk A. J. Adenovirus stimulation of transcription by RNA polymerase III: evidence for an E1A-dependent increase in transcription factor IIIC concentration. EMBO J. 1986 Feb;5(2):343–354. doi: 10.1002/j.1460-2075.1986.tb04218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajchowski D. A., Boeuf H., Kédinger C. The adenovirus-2 early EIIa transcription unit possesses two overlapping promoters with different sequence requirements for EIa-dependent stimulation. EMBO J. 1985 May;4(5):1293–1300. doi: 10.1002/j.1460-2075.1985.tb03775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]