Abstract
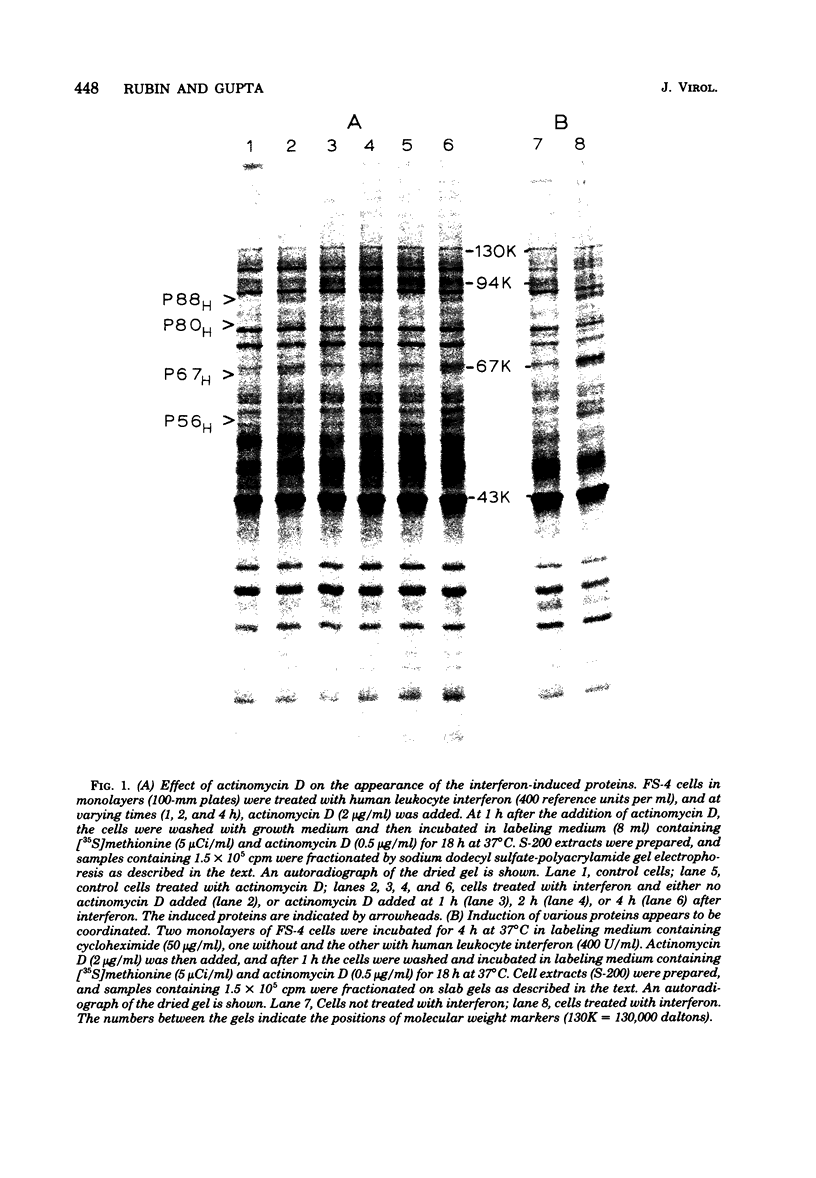
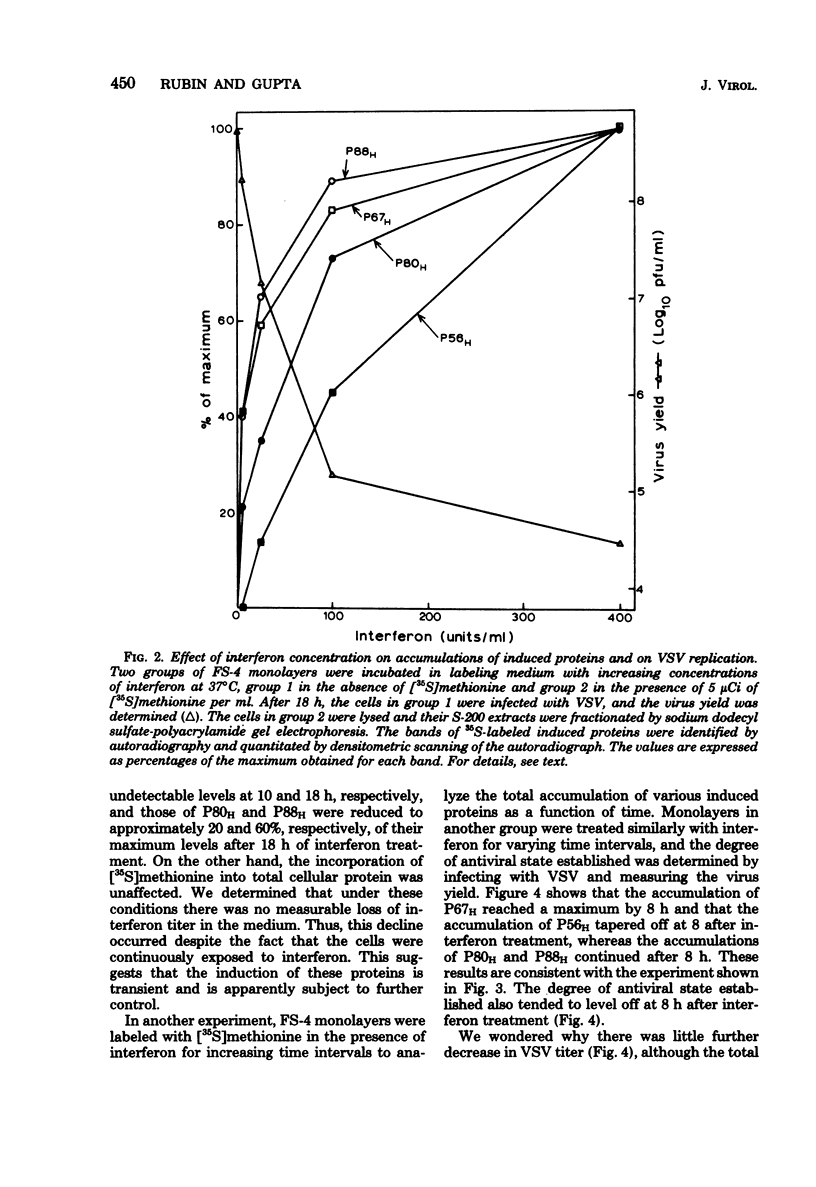
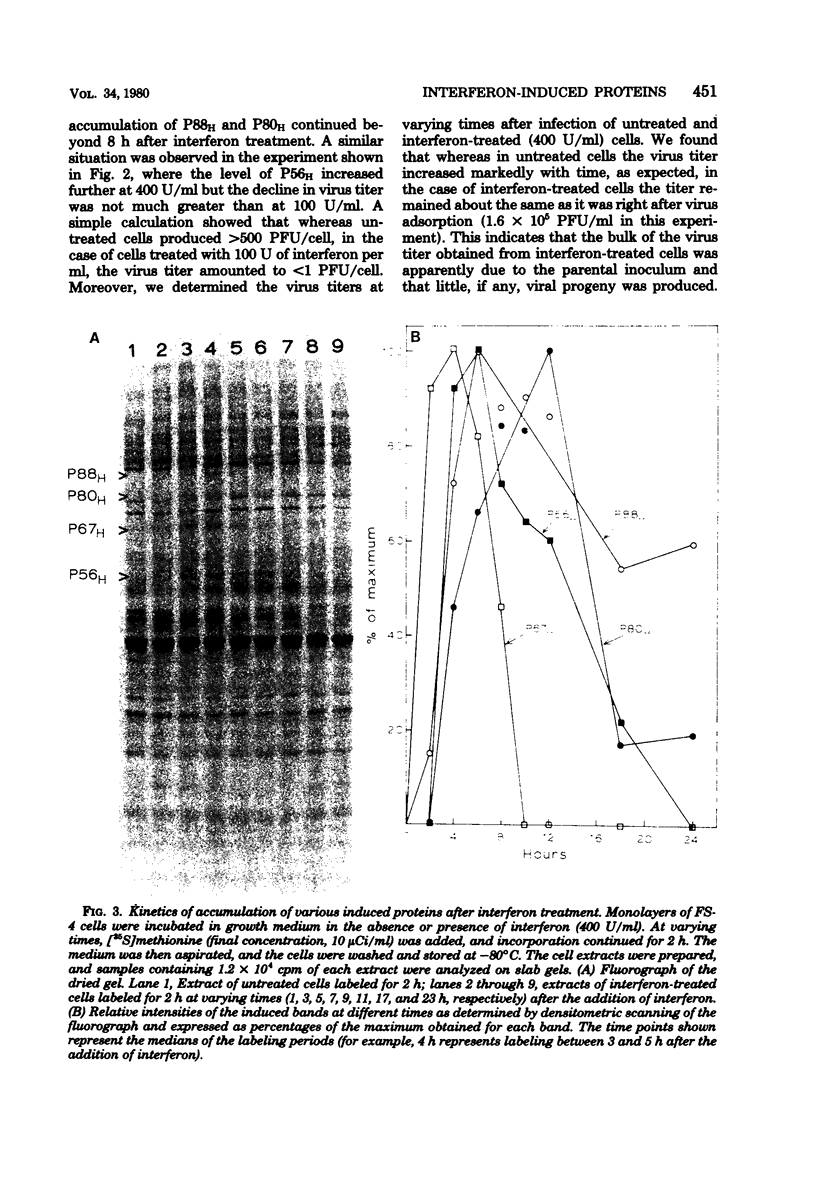
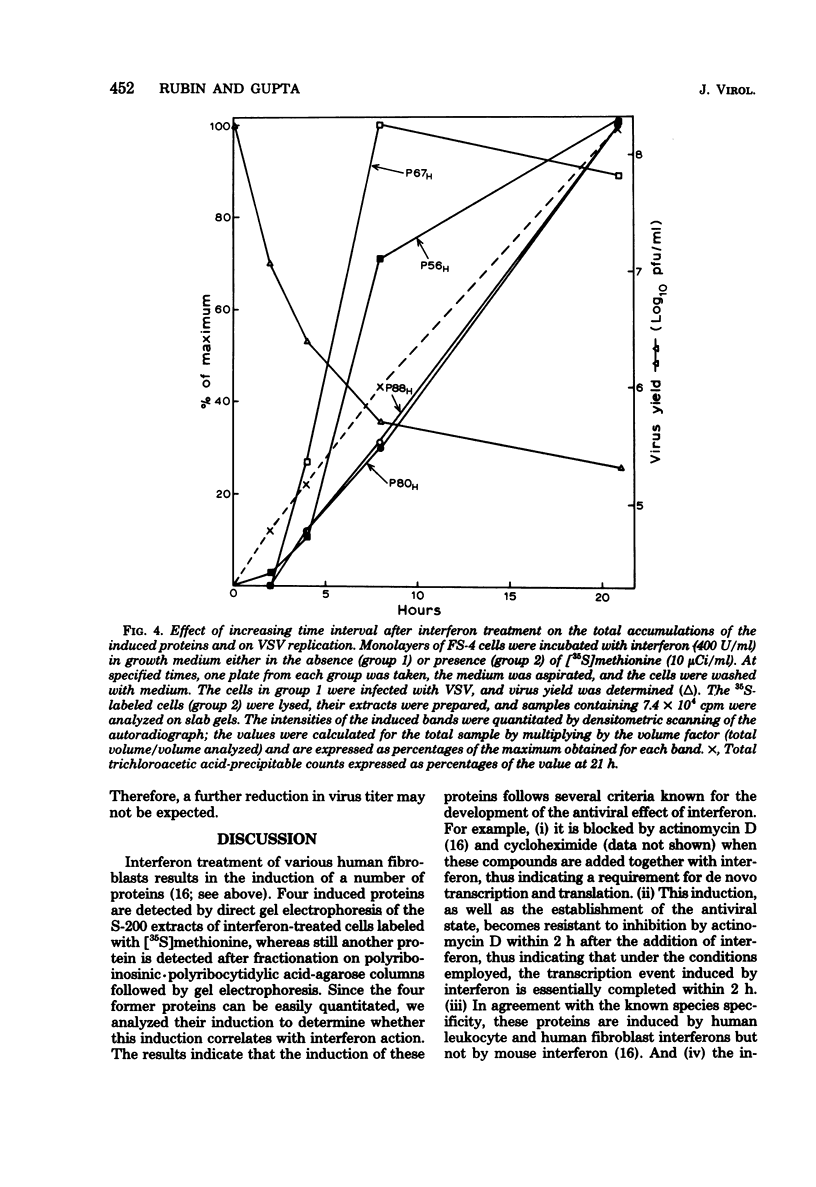
Treatment of human fibroblasts with interferon induces the synthesis of several proteins, as detected by incorporation of [35S]methionine followed by analysis of cell extracts by polyacrylamide gel electrophoresis. The induction of these proteins had features in common with the development of the antiviral effect of interferon, such as (i) sensitivity to actinomycin D and cycloheximide when these compounds were added together with interferon, (ii) insensitivity to actinomycin D if the actinomycin D was added 2 h after the addition of interferon, (iii) similar dependence on interferon concentration, and (iv) species specificity for interferon. When interferon treatment was given in the presence of cycloheximide and actinomycin D was added before the removal of cycloheximide, all four proteins were induced, thus suggesting that their inductions are coordinated. Labeling for 2-h periods at varying time intervals after the addition of interferon revealed that the synthesis of these proteins was induced within a few hours, peaked at different time intervals, and was soon followed by a marked decline, suggesting that the mRNA's for these proteins have short half-lives. Moreover, this decline occurred despite the fact that the cells were continuously exposed to interferon, and there was no measurable loss of interferon activity in the medium. This suggests that the induction of these proteins is transient and is apparently subject to further control.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., Maroney P. A., West D. K. 2'5'Oligo(A) polymerase activity and inhibition of viral RNA synthesis in interferon-treated HeLa cells. Biochemistry. 1979 May 1;18(9):1765–1770. doi: 10.1021/bi00576a020. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Ball L. A. Induction of 2'5'-oligoadenylate synthetase activity and a new protein by chick interferon. Virology. 1979 Apr 30;94(2):282–296. doi: 10.1016/0042-6822(79)90462-8. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Nuclease activation by double-stranded RNA and by 2',5'-oligoadenylate in extracts of interferon-treated chick cells. Virology. 1979 Mar;93(2):348–356. doi: 10.1016/0042-6822(79)90239-3. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Oligonucleotide inhibitor of protein synthesis made in extracts of interferon-treated chick embryo cells: comparison with the mouse low molecular weight inhibitor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1167–1171. doi: 10.1073/pnas.75.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman B., Vilcek J. Cellular binding characteristics of human interferon. Virology. 1974 Feb;57(2):378–386. doi: 10.1016/0042-6822(74)90177-9. [DOI] [PubMed] [Google Scholar]
- Chernajovsky Y., Kimchi A., Schmidt A., Zilberstein A., Revel M. Differential effects of two interferon-induced translational inhibitors on initiation of protein synthesis. Eur J Biochem. 1979 May 2;96(1):35–41. doi: 10.1111/j.1432-1033.1979.tb13010.x. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- De Ley M., Billiau A., De Somer P. Interferon-induced synthesis of a 63,000 dalton protein in mouse cells. Biochem Biophys Res Commun. 1979 Jul 27;89(2):701–705. doi: 10.1016/0006-291x(79)90686-7. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Broeze R. J., Lengyel P. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature. 1979 Jun 7;279(5713):523–525. doi: 10.1038/279523a0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Sen G. C., Dubois M. F., Ratner L., Slattery E., Lengyel P. Interferon action: two distinct pathways for inhibition of protein synthesis by double-stranded RNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5893–5897. doi: 10.1073/pnas.75.12.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Interferon binding: the first step in establishment of antiviral activity. Science. 1967 Jun 30;156(3783):1760–1761. doi: 10.1126/science.156.3783.1760. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Sonnabend J. A. Inhibition of interferon action by puromycin. J Immunol. 1965 Oct;95(4):696–703. [PubMed] [Google Scholar]
- Gupta S. L., Rubin B. Y., Holmes S. L. Interferon action: induction of specific proteins in mouse and human cells by homologous interferons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4817–4821. doi: 10.1073/pnas.76.10.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. L. Specific protein phosphorylation in interferon-treated uninfected and virus-infected mouse L929 cells: enhancement by double-stranded RNA. J Virol. 1979 Jan;29(1):301–311. doi: 10.1128/jvi.29.1.301-311.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G., Brown R. E., Kerr I. M. Synthesis of low molecular weight inhibitor of protein synthesis with enzyme from interferon-treated cells. Nature. 1977 Aug 11;268(5620):537–540. doi: 10.1038/268537a0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E., Jr, Korant B. D. Fibroblast interferon induces synthesis of four proteins in human fibroblast cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1824–1827. doi: 10.1073/pnas.76.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Falcoff E., Falcoff R. Dual action of double-stranded RNA inhibiting protein synthesis in extracts of interferon-treated mouse L cells. Translation is impaired at the level of initiation and by mRNA degradation. Eur J Biochem. 1978 May 16;86(2):497–509. doi: 10.1111/j.1432-1033.1978.tb12333.x. [DOI] [PubMed] [Google Scholar]
- Radke K. L., Colby C., Kates J. R., Krider H. M., Prescott D. M. Establishment and maintenance of the interferon-induced antiviral state: studies in enucleated cells. J Virol. 1974 Mar;13(3):623–630. doi: 10.1128/jvi.13.3.623-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Wiegand R. C., Farrell P. J., Sen G. C., Cabrer B., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Fractionation of the endonucleaseINT system into two macromolecular components; role of a small molecule in nuclease activation. Biochem Biophys Res Commun. 1978 Apr 14;81(3):947–954. doi: 10.1016/0006-291x(78)91443-2. [DOI] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Joklik W. K. A protein synthesizing system from interferon-treated cells that discriminates between cellular and viral messenger RNAs. Virology. 1974 Apr;58(2):476–491. doi: 10.1016/0042-6822(74)90082-8. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci U S A. 1979 Feb;76(2):600–604. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, De Clercq E., De Somer P. Recovery of cell-bound interferon. J Virol. 1972 Oct;10(4):707–712. doi: 10.1128/jvi.10.4.707-712.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. Inhibition of interferon action by actinomycin. Biochem Biophys Res Commun. 1964;14:447–451. doi: 10.1016/0006-291x(64)90084-1. [DOI] [PubMed] [Google Scholar]
- Young C. S., Pringle C. R., Follett E. A. Action of interferon in enucleated cells. J Virol. 1975 Feb;15(2):428–429. doi: 10.1128/jvi.15.2.428-429.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Federman P., Shulman L., Revel M. Specific phosphorylation in vitro of a protein associated with ribosomes of interferon-treated mouse L cells. FEBS Lett. 1976 Sep 15;68(1):119–124. doi: 10.1016/0014-5793(76)80418-8. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Kimchi A., Schmidt A., Revel M. Isolation of two interferon-induced translational inhibitors: a protein kinase and an oligo-isoadenylate synthetase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4734–4738. doi: 10.1073/pnas.75.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]