Abstract
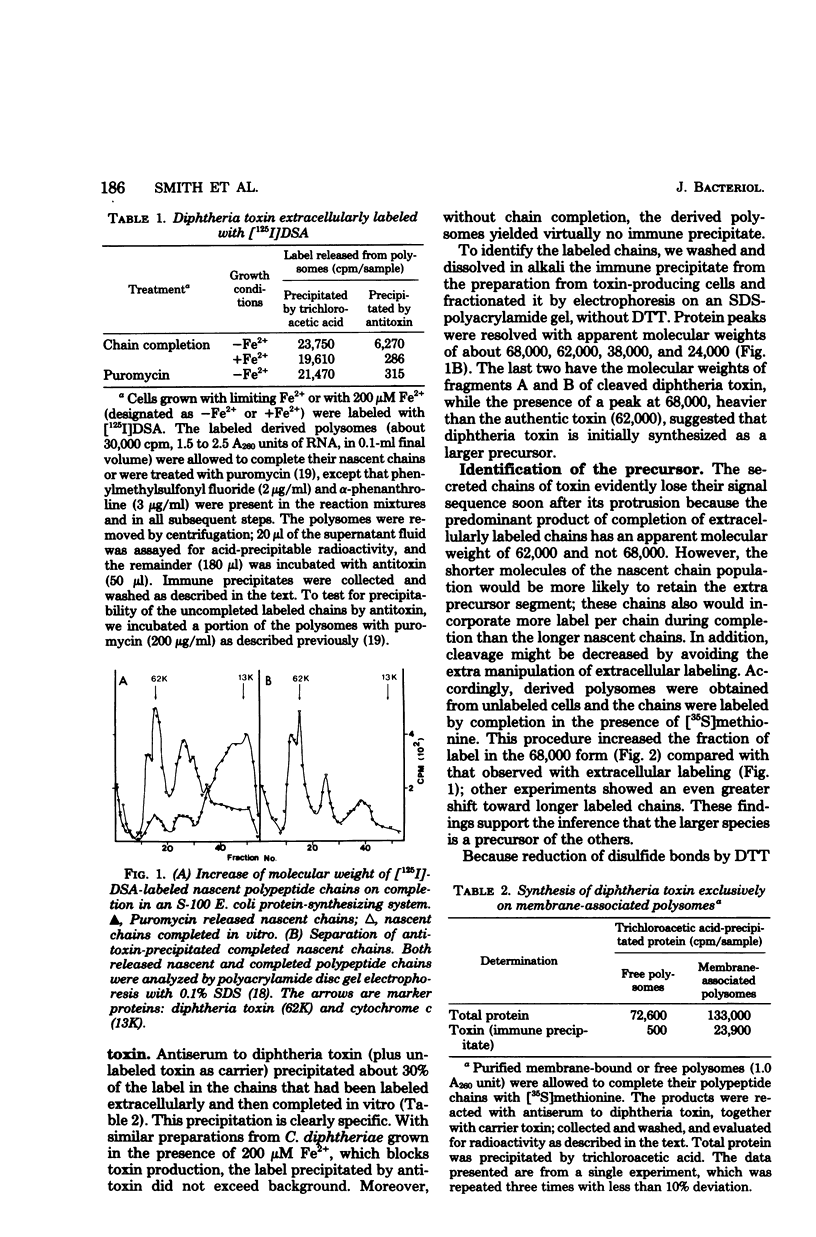
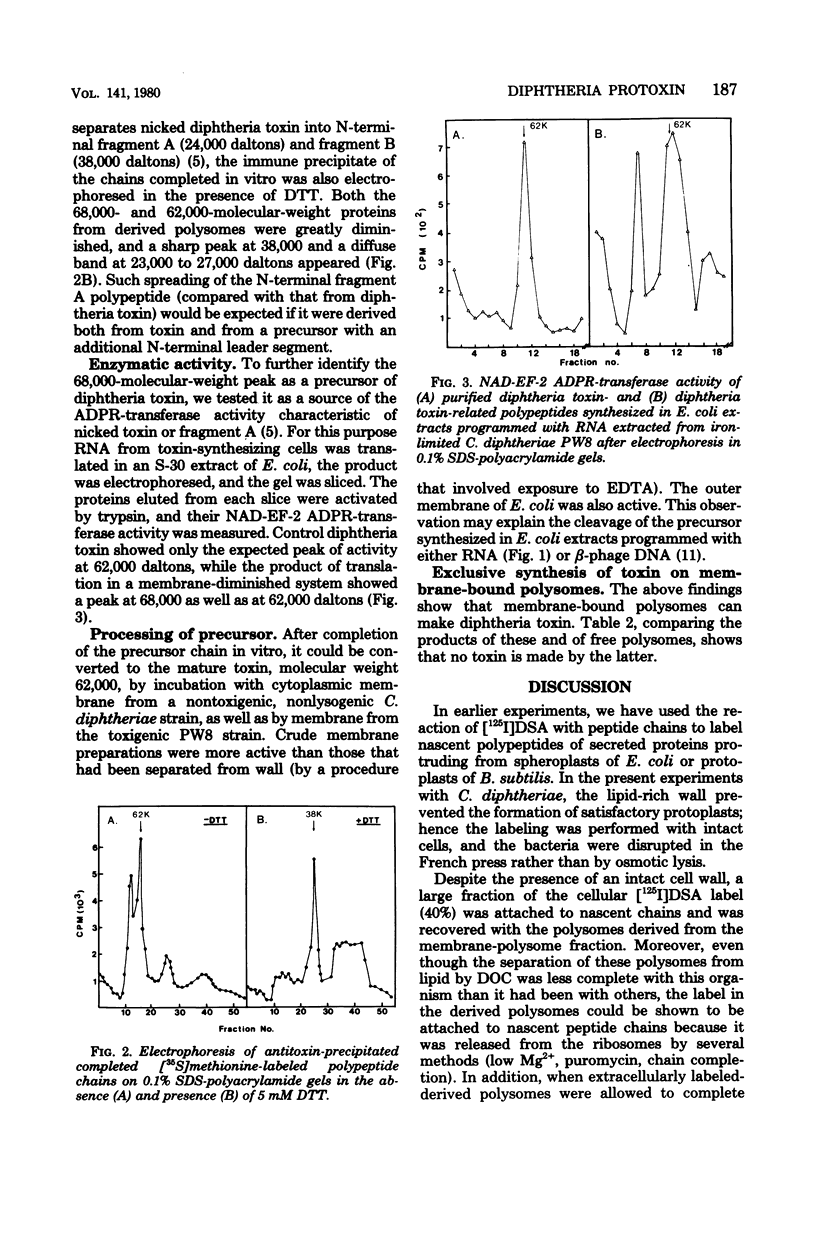
By extracellular labeling of peptides of intact Corynebacterium diphtheriae, followed by fractionation of the cells and chain completion by isolated polysomes, it is shown that diphtheria toxin is formed and secreted cotranslationally by membrane-bound polysomes; free polysomes from none. Moreover, when the chains on these polysomes were completed in vitro, in the absence of membrane they were found to include not only diphtheria toxin of a molecular weight of 62,000, but also a larger precursor of a molecular weight of 68,000. The precursor was identified by several properties: immune precipitation; conversion into toxin fragments A and B; adenosine diphosphate ribosyl-transferase activity after activation with trypsin; and cleavage to 62,000 daltons by membrane enzymes. The precursor yields an N-terminal A fragment with a broadened molecular weight distribution, compared with that from authentic toxin, thus supporting the expectation that the extra segment of the precursor is N-terminal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARDSDALE W. L., PAPPENHEIMER A. M., Jr Phage-host relationships in nontoxigenic and toxigenic diphtheria bacilli. J Bacteriol. 1954 Feb;67(2):220–232. doi: 10.1128/jb.67.2.220-232.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P., Silverman M. S., Pappenheimer A. M., Jr, Vernon W. B. Binding of triton X-100 to diphtheria toxin, crossreacting material 45, and their fragments. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4449–4453. doi: 10.1073/pnas.73.12.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr Structure-activity relationships in diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1492–1495. [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Murphy J. R., Michel J. L., Teng M. Evidence that the regulation of diphtheria toxin production is directed at the level of transcription. J Bacteriol. 1978 Aug;135(2):511–516. doi: 10.1128/jb.135.2.511-516.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Pappenheimer A. M., Jr, de Borms S. T. Synthesis of diphtheria tox-gene products in Escherichia coli extracts. Proc Natl Acad Sci U S A. 1974 Jan;71(1):11–15. doi: 10.1073/pnas.71.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Josefsson L. G. Precursors of three exported proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1209–1212. doi: 10.1073/pnas.75.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Davis B. D. Extracellular labeling of growing secreted polypeptide chains in Bacillus subtilis with diazoiodosulfanilic acid. Biochemistry. 1979 Jan 9;18(1):198–202. doi: 10.1021/bi00568a030. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Thompson R. C., Davis B. D. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. doi: 10.1073/pnas.74.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Wallace B. J., Herzog E. L., Davis B. D. Properties of initiation-free polysomes of Escherichia coli. Biochemistry. 1973 Feb;12(4):609–615. doi: 10.1021/bi00728a007. [DOI] [PubMed] [Google Scholar]
- Uchida T., Yoneda M. Evidence for the association of membrane with the site of toxin synthesis in Corynebacterium diphtheriae. Biochim Biophys Acta. 1967 Aug 22;145(1):210–213. doi: 10.1016/0005-2787(67)90681-8. [DOI] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]


