Abstract
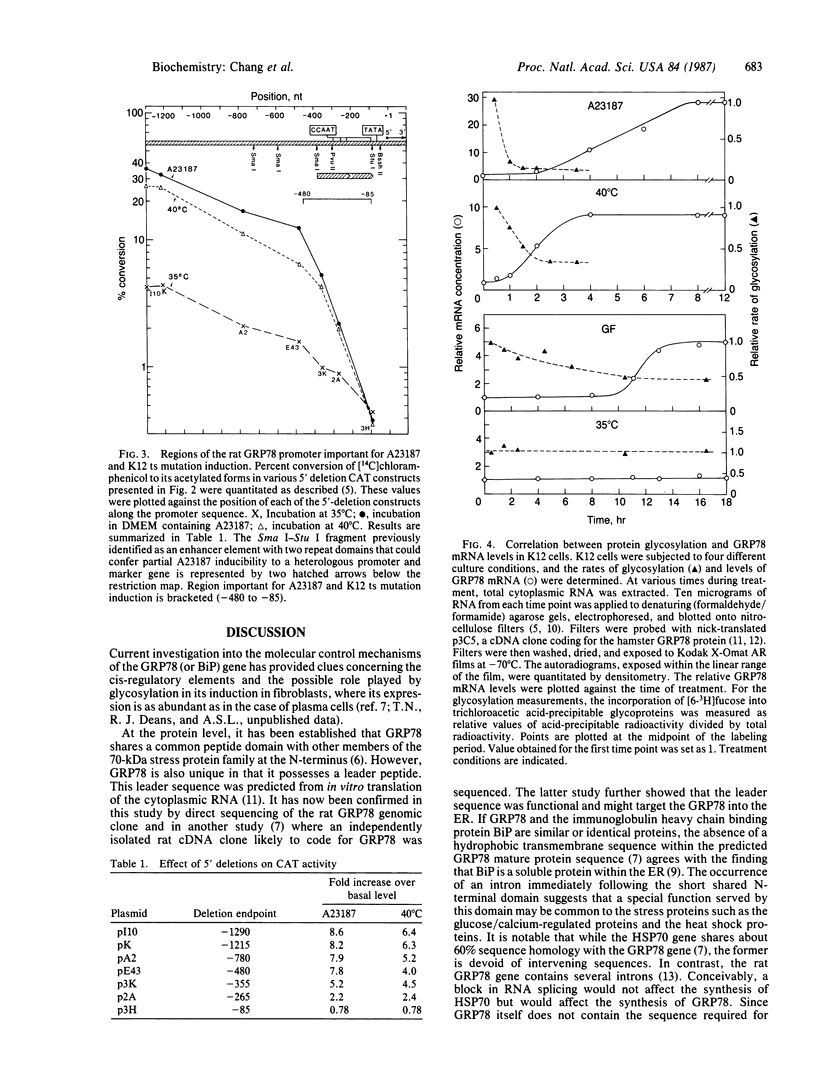
The 78-kDa glucose-regulated protein GRP78 is a stress-inducible protein ubiquitously expressed in animal cells. In this paper we show that the first exon of this endoplasmic reticulum-localized protein consists of an 18 amino acid leader sequence rich in hydrophobic residues, followed by a highly acidic mature N-terminus and an 11 amino acid domain that is shared by members of the 70-kDa heat shock protein family. The end of this shared domain also marks the beginning of the first intron of this gene. A DNA region upstream of the promoter element important for induction by calcium ionophore and by a temperature-sensitive mutation was identified by deletion analysis. Our results indicate that a region spanning from 85 to 480 nucleotides upstream of the major transcription initiation site is important for both induction conditions. With evidence suggesting that perturbations in protein glycosylation may be one of the common stimuli involved in transcription activation of the GRPs, we measured the rate of glycosylation during A23187, glucose starvation, and temperature-shift induced conditions. The inverse correlation observed between the rate of glycosylation and the steady-state level of the GRP78 transcripts lends support to this hypothesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attenello J. W., Lee A. S. Regulation of a hybrid gene by glucose and temperature in hamster fibroblasts. Science. 1984 Oct 12;226(4671):187–190. doi: 10.1126/science.6484570. [DOI] [PubMed] [Google Scholar]
- Bekesi J. G., Winzler R. J. The metabolism of plasma glycoproteins. Studies on the incorporation of L-fucose-1-14-C into tissue and serum in the normal rat. J Biol Chem. 1967 Sep 10;242(17):3873–3879. [PubMed] [Google Scholar]
- Bienz M., Pelham H. R. Heat shock regulatory elements function as an inducible enhancer in the Xenopus hsp70 gene and when linked to a heterologous promoter. Cell. 1986 Jun 6;45(5):753–760. doi: 10.1016/0092-8674(86)90789-0. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E. Newcastle disease virus stimulates the cellular accumulation of stress (heat shock) mRNAs and proteins. J Virol. 1982 Nov;44(2):703–707. doi: 10.1128/jvi.44.2.703-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. S., Bell J., Ting J. Biochemical characterization of the 94- and 78-kilodalton glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1984 Apr 10;259(7):4616–4621. [PubMed] [Google Scholar]
- Lee A. S., Delegeane A. M., Baker V., Chow P. C. Transcriptional regulation of two genes specifically induced by glucose starvation in a hamster mutant fibroblast cell line. J Biol Chem. 1983 Jan 10;258(1):597–603. [PubMed] [Google Scholar]
- Lee A. S., Delegeane A., Scharff D. Highly conserved glucose-regulated protein in hamster and chicken cells: preliminary characterization of its cDNA clone. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4922–4925. doi: 10.1073/pnas.78.8.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. S. The accumulation of three specific proteins related to glucose-regulated proteins in a temperature-sensitive hamster mutant cell line K12. J Cell Physiol. 1981 Jan;106(1):119–125. doi: 10.1002/jcp.1041060113. [DOI] [PubMed] [Google Scholar]
- Lee A. S., Wells S., Kim K. S., Scheffler I. E. Enhanced synthesis of the glucose/calcium-regulated proteins in a hamster cell mutant deficient in transfer of oligosaccharide core to polypeptides. J Cell Physiol. 1986 Dec;129(3):277–282. doi: 10.1002/jcp.1041290302. [DOI] [PubMed] [Google Scholar]
- Lin A. Y., Chang S. C., Lee A. S. A calcium ionophore-inducible cellular promoter is highly active and has enhancerlike properties. Mol Cell Biol. 1986 Apr;6(4):1235–1243. doi: 10.1128/mcb.6.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. Y., Lee A. S. Effect of E1A and E1B viral proteins on the expression of a calcium ionophore-inducible gene and its promoter. Nucleic Acids Res. 1986 Jun 25;14(12):4911–4921. doi: 10.1093/nar/14.12.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. Y., Lee A. S. Induction of two genes by glucose starvation in hamster fibroblasts. Proc Natl Acad Sci U S A. 1984 Feb;81(4):988–992. doi: 10.1073/pnas.81.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melero J. A. Identification of the glucose/glycosylation-regulated proteins as those which accumulate in the temperature-sensitive cell line K12. J Cell Physiol. 1981 Oct;109(1):59–67. doi: 10.1002/jcp.1041090108. [DOI] [PubMed] [Google Scholar]
- Melton D. W., McEwan C., McKie A. B., Reid A. M. Expression of the mouse HPRT gene: deletional analysis of the promoter region of an X-chromosome linked housekeeping gene. Cell. 1986 Jan 31;44(2):319–328. doi: 10.1016/0092-8674(86)90766-x. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Jaworski C., Yamada K. M. Evidence for role of glycoprotein carbohydrates in membrane transport: specific inhibition by tunicamycin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):791–795. doi: 10.1073/pnas.76.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W., Lamb R. A., Choppin P. W. Infection with paramyxoviruses stimulates synthesis of cellular polypeptides that are also stimulated in cells transformed by Rous sarcoma virus or deprived of glucose. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6120–6124. doi: 10.1073/pnas.75.12.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Shiu R. P., Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977 Aug;11(4):941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Resendez E., Jr, Attenello J. W., Grafsky A., Chang C. S., Lee A. S. Calcium ionophore A23187 induces expression of glucose-regulated genes and their heterologous fusion genes. Mol Cell Biol. 1985 Jun;5(6):1212–1219. doi: 10.1128/mcb.5.6.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez E., Jr, Ting J., Kim K. S., Wooden S. K., Lee A. S. Calcium ionophore A23187 as a regulator of gene expression in mammalian cells. J Cell Biol. 1986 Dec;103(6 Pt 1):2145–2152. doi: 10.1083/jcb.103.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe D. H., Read M., Robinson H. Isolation of temperature sensitive mammalian cells by selective detachment. J Cell Physiol. 1973 Dec;82(3):325–331. doi: 10.1002/jcp.1040820302. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenner A. J., Scheffler I. E. Lipid-saccharide intermediates and glycoprotein biosynthesis in a temperature-sensitive Chinese hamster cell mutant. J Cell Physiol. 1979 Feb;98(2):251–266. doi: 10.1002/jcp.1040980202. [DOI] [PubMed] [Google Scholar]
- Tenner A., Zieg J., Scheffler I. E. Glycoprotein synthesis in a temperature-sensitive Chinese hamster cell cycle mutant. J Cell Physiol. 1977 Feb;90(2):145–160. doi: 10.1002/jcp.1040900202. [DOI] [PubMed] [Google Scholar]
- Wang M. L., Lee A. S. Polymerization of vector DNA after transfection into hamster fibroblast cells. Biochem Biophys Res Commun. 1983 Jan 27;110(2):593–601. doi: 10.1016/0006-291x(83)91191-9. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Garrels J. I., Thomas G. P., Lin J. J., Feramisco J. R. Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose- and Ca2+-ionophore-regulated proteins. J Biol Chem. 1983 Jun 10;258(11):7102–7111. [PubMed] [Google Scholar]
- Whelan S. A., Hightower L. E. Differential induction of glucose-regulated and heat shock proteins: effects of pH and sulfhydryl-reducing agents on chicken embryo cells. J Cell Physiol. 1985 Nov;125(2):251–258. doi: 10.1002/jcp.1041250212. [DOI] [PubMed] [Google Scholar]
- Wu B. J., Kingston R. E., Morimoto R. I. Human HSP70 promoter contains at least two distinct regulatory domains. Proc Natl Acad Sci U S A. 1986 Feb;83(3):629–633. doi: 10.1073/pnas.83.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala C. A., Salas-Prato M., Yan W. T., Banjo B., Perdue J. F. In cultured chick embryo fibroblasts the hexose transport components are not the 75 000 and 95 000 dalton polypeptides synthesized following glucose deprivation. Can J Biochem. 1980 Oct;58(10):1179–1188. doi: 10.1139/o80-158. [DOI] [PubMed] [Google Scholar]