Abstract
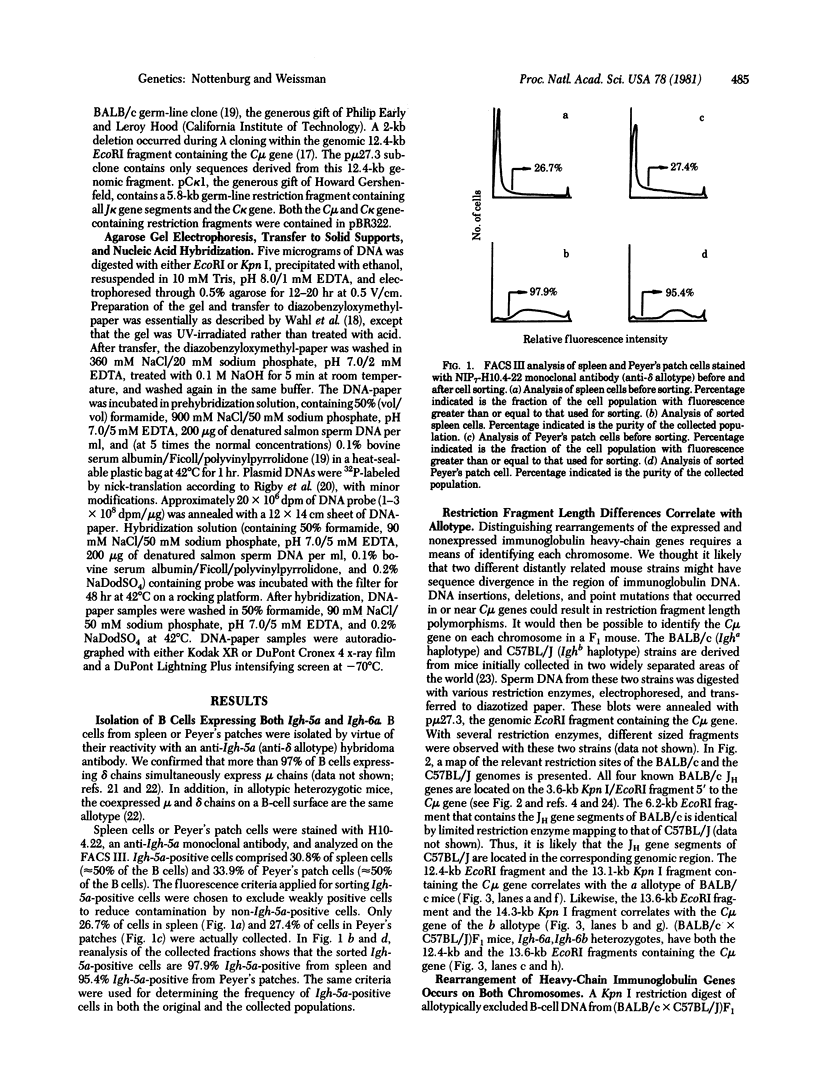
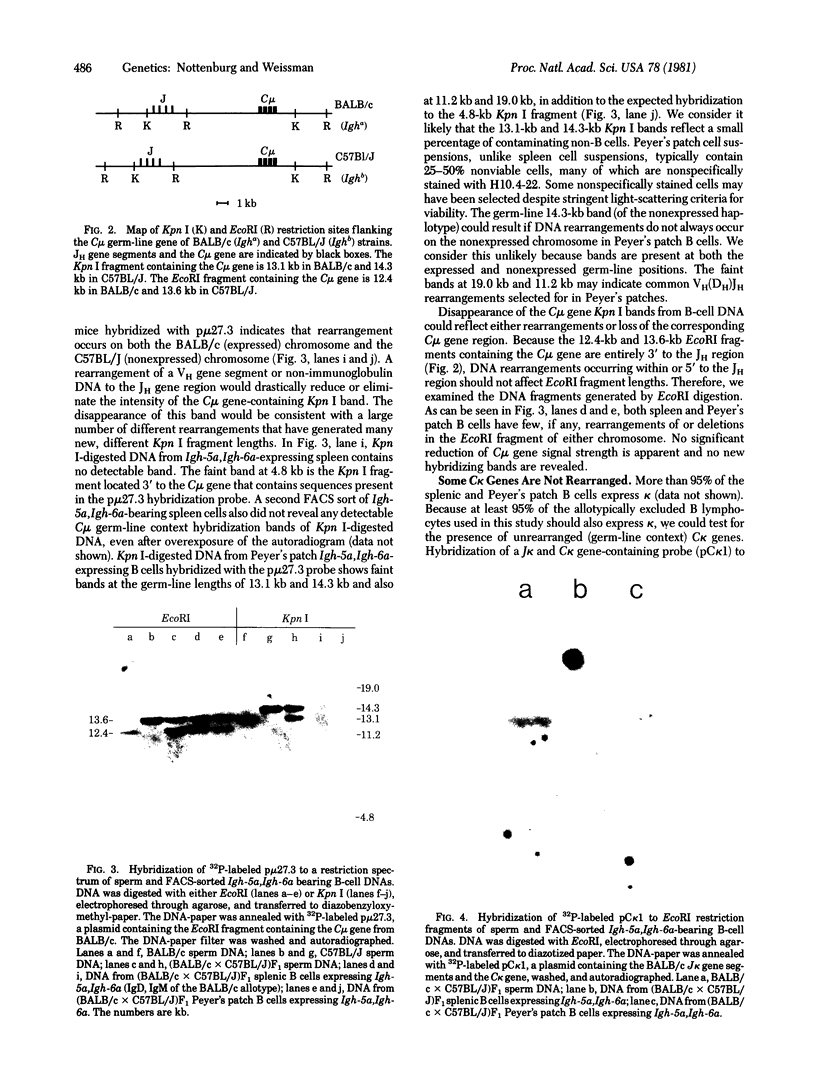
We have examined the organization of heavy-chain immunoglobulin genes on both the expressed and nonexpressed chromosomes of normal B lymphocytes from allotype heterozygous (BALB/c X C57BL/J)F1 mice. The C mu genes of BALB/c mice are on 12.4-kilobase EcoRI and 13.1-kilobase Kpn I restriction fragments, whereas those of C57BL/J mice are on 13.6-kilobase EcoRI and 14.3-kilobase Kpn I restriction fragments, allowing the examination of rearrangements on each chromosome independently. B lymphocytes from spleen and Peyer's patches expressing both IgD and IgM of the BALB/c allotype were isolated with a fluorescence-activated cell sorter. EcoRI and Kpn I restriction digests were hybridized with a C mu gene-containing probe. The C mu gene is present on both chromosomes. DNA rearrangements occur on both the expressed and nonexpressed chromosome within the 3.6-kilobase Kpn I/EcoRI restriction fragment containing the joining (JH) gene locus. We conclude that allelic exclusion of heavy-chain immunoglobulin gene expression is not mediated by JH-region DNA rearrangement of the expressed chromosome only. In contrast, analysis of the C kappa gene region from the same sorted B-cell DNA reveals a substantial quantity of germ-line context DNA. We also demonstrate that the deletions observed on the Eco RI fragment containing the C mu gene in myeloma cells and in C mu gene-containing recombinant DNAs do not usually occur in normally differentiating B lymphocytes and are likely to be confined to myeloma tumor cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Calame K., Rogers J., Early P., Davis M., Livant D., Wall R., Hood L. Mouse Cmu heavy chain immunoglobulin gene segment contains three intervening sequences separating domains. Nature. 1980 Apr 3;284(5755):452–455. doi: 10.1038/284452a0. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Cooper D., Perry R. P. Rearrangement of immunoglobulin heavy chain genes during B-lymphocyte development as revealed by studies of mouse plasmacytoma cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1422–1426. doi: 10.1073/pnas.77.3.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Deletions are associated with somatic rearrangement of immunoglobulin heavy chain genes. Cell. 1980 Jan;19(1):37–51. doi: 10.1016/0092-8674(80)90386-4. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Calame K., Early P. W., Livant D. L., Joho R., Weissman I. L., Hood L. An immunoglobulin heavy-chain gene is formed by at least two recombinational events. Nature. 1980 Feb 21;283(5749):733–739. doi: 10.1038/283733a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dreyer W. J., Bennett J. C. The molecular basis of antibody formation: a paradox. Proc Natl Acad Sci U S A. 1965 Sep;54(3):864–869. doi: 10.1073/pnas.54.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Goding J. W., Layton J. E. Antigen-induced co-capping of IgM and IgD-like receptors on murine B cells. J Exp Med. 1976 Sep 1;144(3):852–857. doi: 10.1084/jem.144.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M., Kemp D. J., Tyler B. M., Adams J. M., Cory S. Intervening sequences divide the gene for the constant region of mouse immunoglobulin mu chains into segments, each encoding a domain. Proc Natl Acad Sci U S A. 1980 Jan;77(1):554–558. doi: 10.1073/pnas.77.1.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho R., Weissman I. L., Early P., Cole J., Hood L. Organization of kappa light chain genes in germ-line and somatic tissue. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1106–1110. doi: 10.1073/pnas.77.2.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho R., Weissman I. L. V-J joining of immunoglobulin kappa genes only occurs on one homologous chromosome. Nature. 1980 Mar 13;284(5752):179–181. doi: 10.1038/284179a0. [DOI] [PubMed] [Google Scholar]
- Loken M. R., Herzenber L. A. Analysis of cell populations with a fluorescence-activated cell sorter. Ann N Y Acad Sci. 1975 Jun 30;254:163–171. doi: 10.1111/j.1749-6632.1975.tb29166.x. [DOI] [PubMed] [Google Scholar]
- Maki R., Traunecker A., Sakano H., Roeder W., Tonegawa S. Exon shuffling generates an immunoglobulin heavy chain gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2138–2142. doi: 10.1073/pnas.77.4.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Pernis B., Chiappino G., Kelus A. S., Gell P. G. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965 Nov 1;122(5):853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Seidman J. G., Leder P., Tonegawa S., Matthyssens G., Weigert M. Transcription of mouse kappa chain genes: implications for allelic exclusion. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1937–1941. doi: 10.1073/pnas.77.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Forster A. Evidence for noncontiguous variable and constant region genes in both germ line and myeloma DNA. Cell. 1978 Feb;13(2):319–327. doi: 10.1016/0092-8674(78)90200-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. The arrangement and rearrangement of antibody genes. Nature. 1978 Dec 21;276(5690):790–795. doi: 10.1038/276790a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Storb U., Arp B. Immunoglobulin gene rearrangements in hybridoma cells. J Immunol. 1980 May;124(5):2071–2076. [PubMed] [Google Scholar]