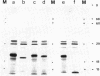
Abstract
Plasmids containing cDNAs for human interferon (IfN) alpha-1, IFN alpha-2, and several hybrids of the two cDNAs, all joined identically to an Escherichia coli lac promoter fragment gave rise, in E. coli, to fused interferons (fIFNs) that had very different target-cell specificities. fIFN alpha-1 had a lower specific activity on human WISH cells than on bovine MDBK cells, while fIFN alpha-2 showed the opposite behavior. fIFN hybrids with the NH2-proximal half of fIFN alpha-2 behaved qualitatively like fIFN alpha-1, and those with the NH2-proximal half of fIFN alpha-2, behaved like fIFN alpha-2. On mouse L929 cells, fIFN alpha-2 was almost inactive, while fIFN alpha-1 showed relatively high activity. In this case, the fIFN hybrids with the COOH-proximal half of IFN alpha-1 showed activity on mouse cells, while the reciprocal hybrid did not. In many cases, the activity spectrum of the hybrids was very different from that of either parent. We propose that the IFN molecule has either two binding sites or two regions constituting the binding site, one in the COOH- and the other in the NH2-proximal half. The experimental findings can be accounted for if the fits of the two sites to their receptor counterparts on different cell lines are independent of one another.
Full text
PDF
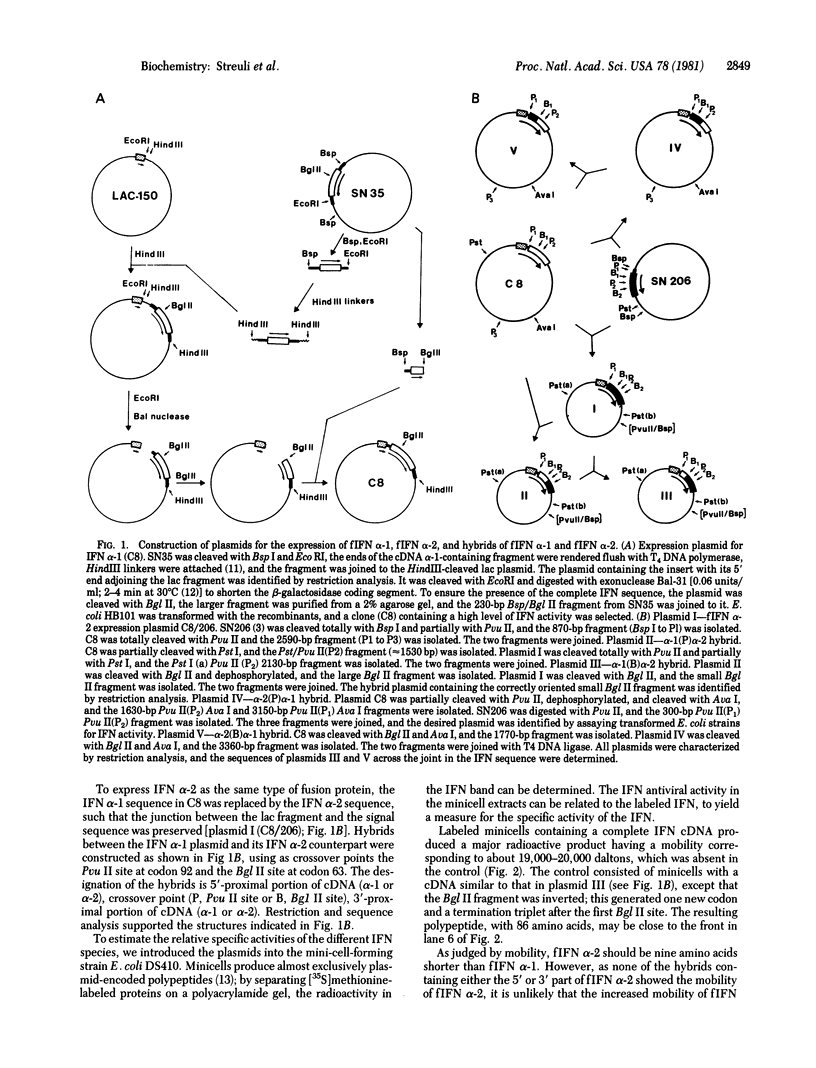



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besancon F., Ankel H., Basu S. Specificity and reversibility of interferon ganglioside interaction. Nature. 1976 Feb 19;259(5544):576–578. doi: 10.1038/259576a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Garoff H., Lehrach H. A subcloning strategy for DNA sequence analysis. Nucleic Acids Res. 1980 Dec 11;8(23):5541–5549. doi: 10.1093/nar/8.23.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H. B., Jr, Ostrander D. A., Hodnett J. L., Legerski R. J., Robberson D. L. Extracellular nucleases of Pseudomonas BAL 31. I. Characterization of single strand-specific deoxyriboendonuclease and double-strand deoxyriboexonuclease activities. Nucleic Acids Res. 1975 Sep;2(9):1459–1492. doi: 10.1093/nar/2.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman E. F., Lee G., Ramos S., Lazo P. S., Kaback H. R., Friedman R. M., Kohn L. D. Relationships of the structure and function of the interferon receptor to hormone receptors and establishment of the antiviral state. Cancer Res. 1978 Nov;38(11 Pt 2):4172–4185. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mantei N., Schwarzstein M., Streuli M., Panem S., Nagata S., Weissmann C. The nucleotide sequence of a cloned human leukocyte interferon cDNA. Gene. 1980 Jun;10(1):1–10. doi: 10.1016/0378-1119(80)90137-7. [DOI] [PubMed] [Google Scholar]
- Masucci M. G., Szigeti R., Klein E., Klein G., Gruest J., Montagnier L., Taira H., Hall A., Nagata S., Weissmann C. Effect of interferon-alpha 1 from E. coli on some cell functions. Science. 1980 Sep 19;209(4463):1431–1435. doi: 10.1126/science.6158096. [DOI] [PubMed] [Google Scholar]
- Mertens G., Reeve J. N. Synthesis of cell envelope components by anucleate cells (minicells) of Bacillus subtilis. J Bacteriol. 1977 Mar;129(3):1198–1207. doi: 10.1128/jb.129.3.1198-1207.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Brack C., Henco K., Schamböck A., Weissmann C. Partial mapping of ten genes of the human interferon- alpha family. J Interferon Res. 1981 Feb;1(2):333–336. doi: 10.1089/jir.1981.1.333. [DOI] [PubMed] [Google Scholar]
- Nagata S., Mantei N., Weissmann C. The structure of one of the eight or more distinct chromosomal genes for human interferon-alpha. Nature. 1980 Oct 2;287(5781):401–408. doi: 10.1038/287401a0. [DOI] [PubMed] [Google Scholar]
- Nagata S., Taira H., Hall A., Johnsrud L., Streuli M., Ecsödi J., Boll W., Cantell K., Weissmann C. Synthesis in E. coli of a polypeptide with human leukocyte interferon activity. Nature. 1980 Mar 27;284(5754):316–320. doi: 10.1038/284316a0. [DOI] [PubMed] [Google Scholar]
- Noel D., Nikaido K., Ames G. F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. doi: 10.1021/bi00586a017. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Dickerson R. E., Boyer H. W., Riggs A. D., Itakura K. Chemical synthesis of restriction enzyme recognition sites useful for cloning. Science. 1977 Apr 8;196(4286):177–180. doi: 10.1126/science.847463. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Sarkar F. H., Taira H., Hall A., Nagata S., Weissmann C. Comparisons of several biological and physicochemical properties of human leukocyte interferons produced my human leukocytes and by E. coli. Gene. 1980 Nov;11(3-4):181–186. doi: 10.1016/0378-1119(80)90058-x. [DOI] [PubMed] [Google Scholar]
- Streuli M., Nagata S., Weissmann C. At least three human type alpha interferons: structure of alpha 2. Science. 1980 Sep 19;209(4463):1343–1347. doi: 10.1126/science.6158094. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Mantei N., Schwarzstein M., Nagata S., Muramatsu M., Weissmann C. Human leukocyte and fibroblast interferons are structurally related. Nature. 1980 Jun 19;285(5766):547–549. doi: 10.1038/285547a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Boll W., Mantei N., Lonsdale D., Weissmann C. Hybrid plasmids containing an active thymidine kinase gene of Herpes simplex virus 1. Nucleic Acids Res. 1979 Oct 25;7(4):859–877. doi: 10.1093/nar/7.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong W. W., Zweers A., Cohen L. H. Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes. Biochem Biophys Res Commun. 1978 May 30;82(2):532–539. doi: 10.1016/0006-291x(78)90907-5. [DOI] [PubMed] [Google Scholar]