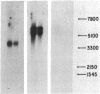
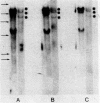
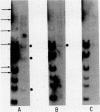
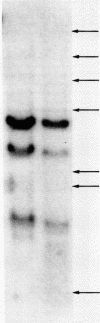
Abstract
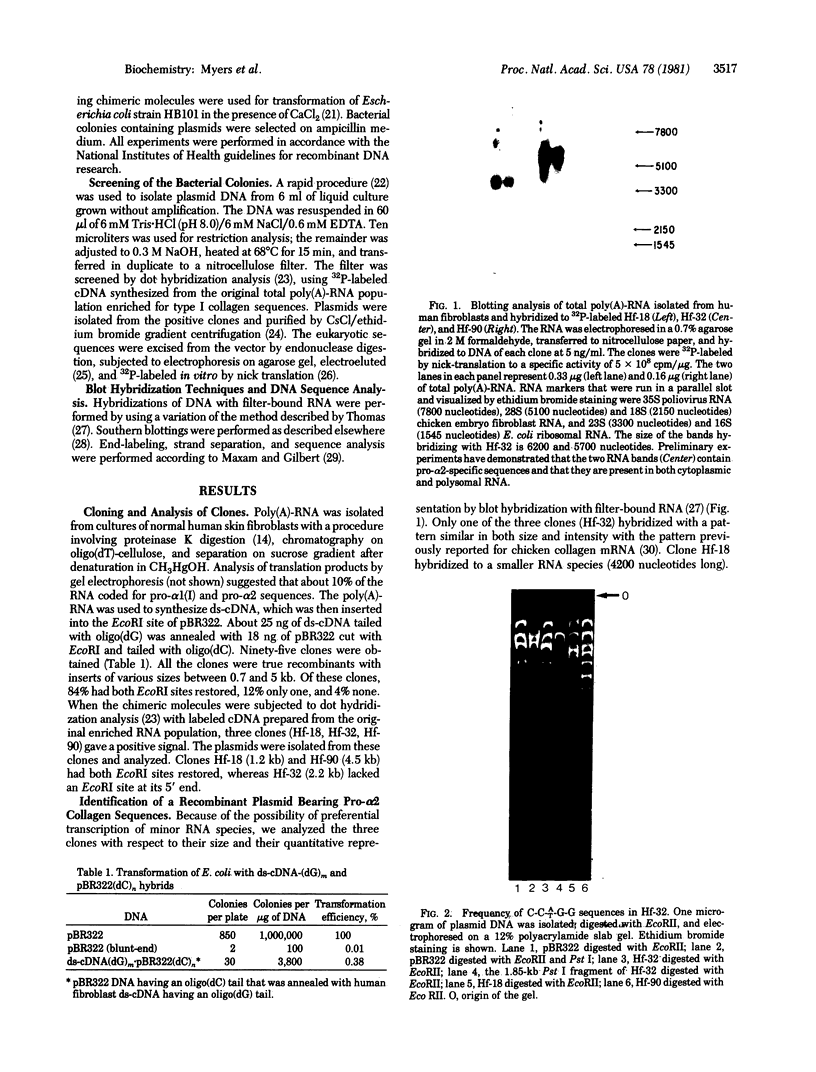
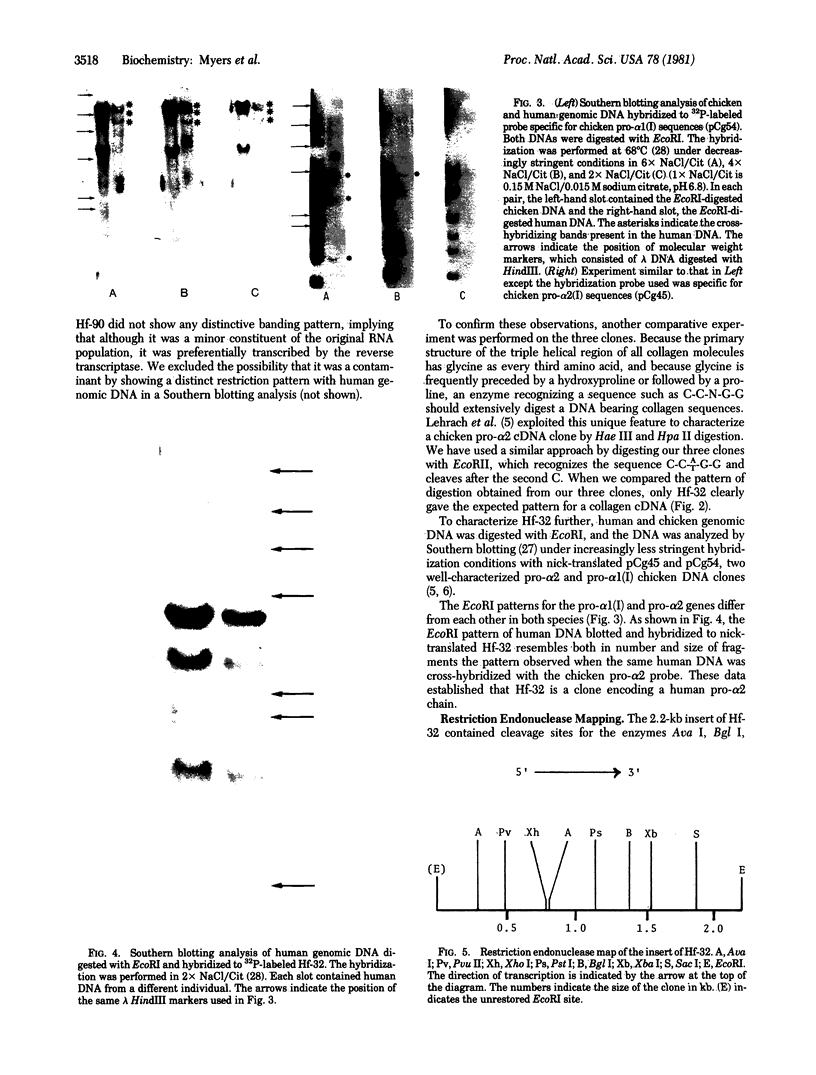
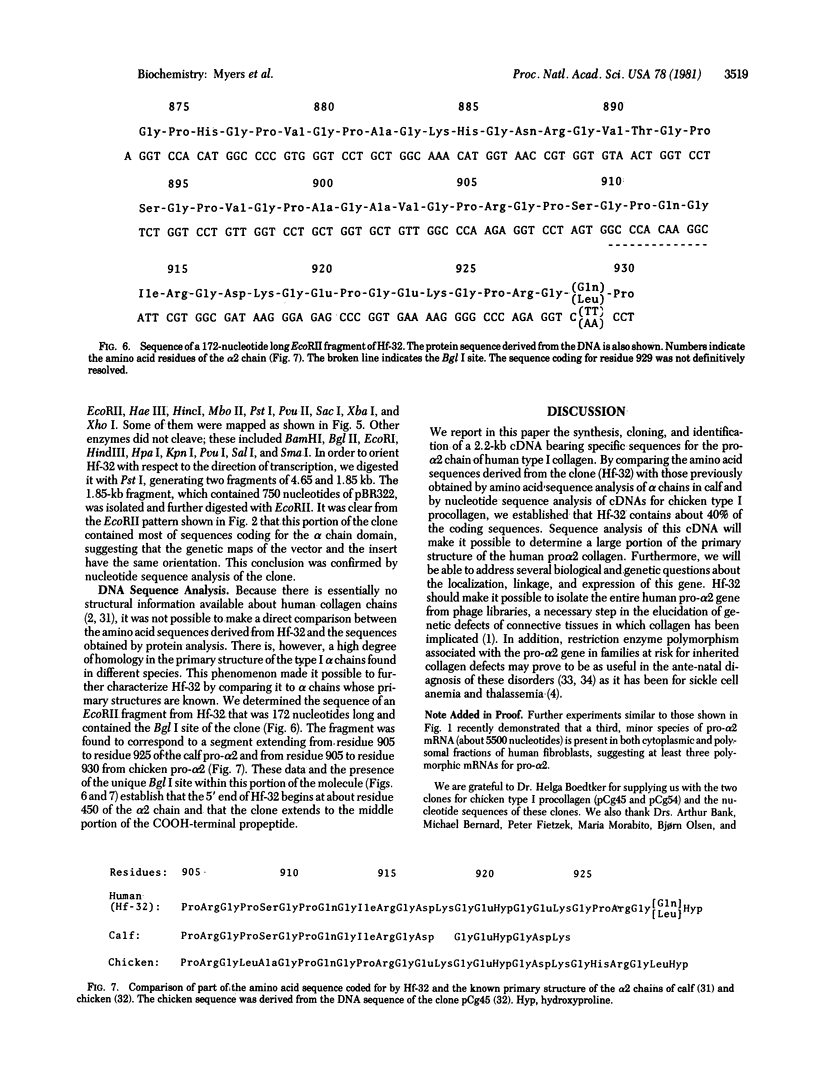
Poly(A)-RNA enriched for type I procollagen sequences was isolated from normal human fibroblasts and used as template to synthesize double-stranded cDNA with avian myeloblastosis virus (AMV) reverse transcriptase. After the ends had been blunted with nuclease S1 and dGMP tails had been added with terminal deoxynucleotidyltransferase, the double-stranded cDNA was annealed with pBR322 DNA that had previously been cleaved with EcoRI, blunted with AMV reverse transcriptase, and dCMP-tailed with terminal deoxynucleotidyltransferase. The chimeric molecule was used to transform Escherichia coli strain HB101. Ninety-five recombinant clones were obtained and screened by dot hybridization analysis using 32P-labeled cDNA synthesized from the original poly(A)-RNA collagen-enriched population. Three positive clones were isolated and further characterized by blot hybridization techniques and by EcoRII digestion. One clone with an insert of 2.2 kilobases was shown to contain sequences encoding for the pro-alpha 2 chain of human type I procollagen. DNA sequence analysis of a 172-nucleotide fragment demonstrated that the cloned cDNA extends from amino acid position 450 of the alpha 2 chain to the middle of the COOH-terminal propeptide.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Alwine J. C., de Crombrugghe B., Pastan I. Use of recombinant plasmids to characterize collagen RNAs in normal and transformed chick embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):4935–4938. [PubMed] [Google Scholar]
- Avvedimento V. E., Vogeli G., Yamada Y., Maizel J. V., Jr, Pastan I., de Crombrugghe B. Correlation between splicing sites within an intron and their sequence complementarity with U1 RNA. Cell. 1980 Oct;21(3):689–696. doi: 10.1016/0092-8674(80)90432-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Boyd C. D., Tolstoshev P., Schafer M. P., Trapnell B. C., Coon H. C., Kretschmer P. J., Nienhuis A. W., Crystal R. G. Isolation and characterization of a 15-kilobase genomic sequence coding for part of the Pro alpha 2 chain of sheep type I collagen. J Biol Chem. 1980 Apr 10;255(7):3212–3220. [PubMed] [Google Scholar]
- Burnett W., Rosenbloom J. Isolation and translation of elastin mRNA from chick aorta. Biochem Biophys Res Commun. 1979 Feb 14;86(3):478–484. doi: 10.1016/0006-291x(79)91739-x. [DOI] [PubMed] [Google Scholar]
- Fuller F., Boedtker H. Sequence determination and analysis of the 3' region of chicken pro-alpha 1(I) and pro-alpha 2(I) collagen messenger ribonucleic acids including the carboxy-terminal propeptide sequences. Biochemistry. 1981 Feb 17;20(4):996–1006. doi: 10.1021/bi00507a054. [DOI] [PubMed] [Google Scholar]
- Hofmann H., Fietzek P. P., Kühn K. The role of polar and hydrophobic interactions for the molecular packing of type I collagen: a three-dimensional evaluation of the amino acid sequence. J Mol Biol. 1978 Oct 25;125(2):137–165. doi: 10.1016/0022-2836(78)90342-x. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Frischauf A. M., Hanahan D., Wozney J., Fuller F., Boedtker H. Construction and characterization of pro alpha 1 collagen complementary deoxyribonucleic acid clones. Biochemistry. 1979 Jul 10;18(14):3146–3152. doi: 10.2196/47873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Frischauf A. M., Hanahan D., Wozney J., Fuller F., Crkvenjakov R., Boedtker H., Doty P. Construction and characterization of a 2.5-kilobase procollagen clone. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5417–5421. doi: 10.1073/pnas.75.11.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears J. G., Ramirez F., Leibowitz D., Bank A. Organization of human delta--and beta-globin genes in cellular DNA and the presence of intragenic inserts. Cell. 1978 Sep;15(1):15–23. doi: 10.1016/0092-8674(78)90079-x. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Ramirez F., Kacian D. L., Flood M., Spiegelman S. A simple purification of avian myeloblastosis virus reverse transcriptase for full-length transcription of 35 S RNA. Anal Biochem. 1980 Jan 1;101(1):88–96. doi: 10.1016/0003-2697(80)90044-5. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S., Kacian D. L. Synthesis of full-length DNA copies of avian myeloblastosis virus RNA in high yields. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2840–2843. doi: 10.1073/pnas.74.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S. Sodium pyrophosphate inhibition of RNA.DNA hybrid degradation by reverse transcriptase. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5329–5333. doi: 10.1073/pnas.75.11.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo H., Vogeli G., Mudryj M., Avvedimento V. E., Sullivan M., Pastan I., de Crombrugghe B. Isolation and characterization of overlapping genomic clones covering the chicken alpha 2 (type I) collagen gene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7059–7063. doi: 10.1073/pnas.77.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Improvements in immunoprecipitation of specific messenger RNA. Isolation of highly purified conalbumin mRNA in high yield. Eur J Biochem. 1979 Nov 1;101(1):271–282. doi: 10.1111/j.1432-1033.1979.tb04240.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F., Mears J. G., Bank A. The molecular basis of disorders of human hemoglobin synthesis. Mol Cell Biochem. 1980 Aug 16;31(3):133–145. doi: 10.1007/BF00225847. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Mach B. Cloning and amplification of rabbit alpha- and beta-globin gene sequences into Escherichia coli plasmids. J Biol Chem. 1977 Apr 10;252(7):2209–2217. [PubMed] [Google Scholar]
- Sobel M. E., Yamamoto T., Adams S. L., DiLauro R., Avvedimento V. E., de Crombrugghe B., Pastan I. Construction of a recombinant bacterial plasmid containing a chick pro-alpha2 collagen gene sequence. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5846–5850. doi: 10.1073/pnas.75.12.5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney J., Hanahan D., Morimoto R., Boedtker H., Doty P. Fine structural analysis of the chicken pro alpha 2 collagen gene. Proc Natl Acad Sci U S A. 1981 Feb;78(2):712–716. doi: 10.1073/pnas.78.2.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Avvedimento V. E., Mudryj M., Ohkubo H., Vogeli G., Irani M., Pastan I., de Crombrugghe B. The collagen gene: evidence for its evolutinary assembly by amplification of a DNA segment containing an exon of 54 bp. Cell. 1980 Dec;22(3):887–892. doi: 10.1016/0092-8674(80)90565-6. [DOI] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]