Abstract
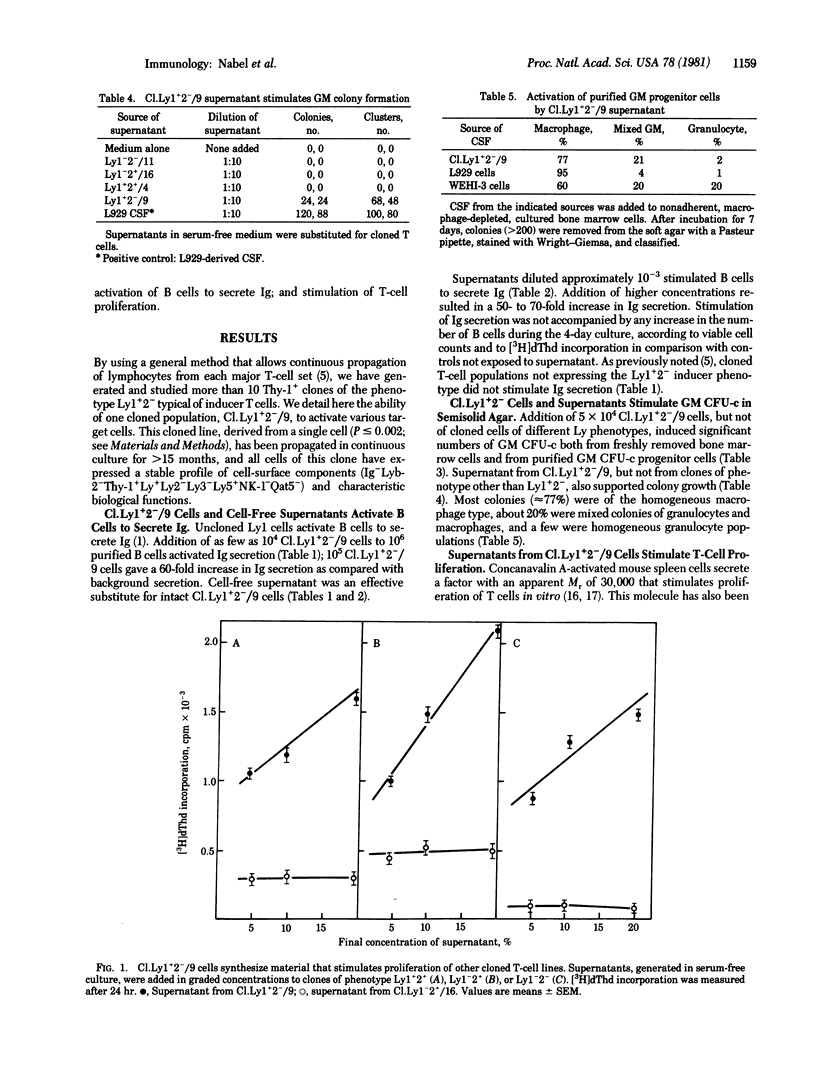
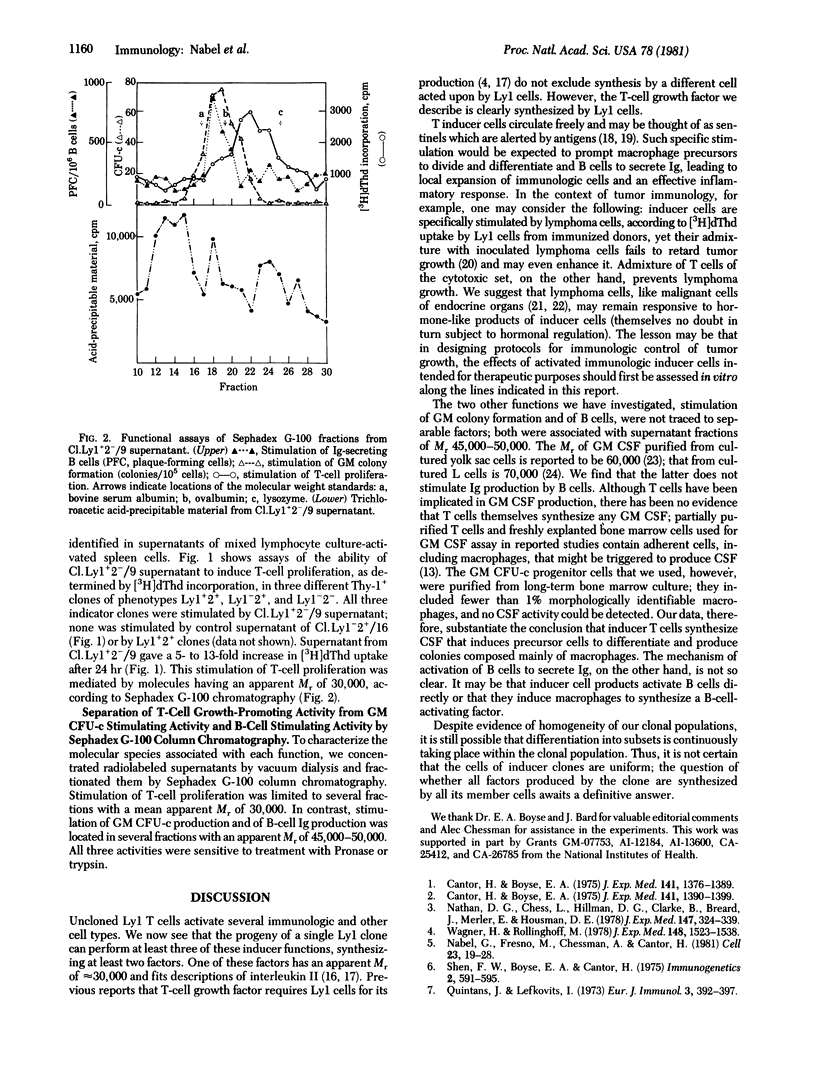
The mouse T-cell clone Ly1+2(-)/9, belonging to the Ly1 set, displays the following functions in vitro: (i) augmentation of immunoglobulin output by B cells; (ii) stimulation of bone marrow cells to produce colonies composed of granulocytes, macrophages, or both; and (iii) proliferative stimulation of T-cell clones belonging to other Ly sets. These functions are induced by Ly1+2(-)/9 cells themselves and by supernatants of Ly1+2(-)/9 cultures and are not evinced by tested clones belonging to other Ly sets. The agent or agents responsible for colony formation and for B-cell stimulation had an apparent molecular weight of 45,000-50,000 and could not be physically separated. The T-cell stimulating agent(s) had an apparent molecular weight of 30,000 and could be separated from the agent(s) that acts upon colony formation and B cells. Thus, clone LY1+2(-)/9 produces at least two soluble products that induce or augment activities of at least three other differently programmed cell sets.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975 Jun 1;141(6):1390–1399. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H. Control of the immune system by inhibitor and inducer T lymphocytes. Annu Rev Med. 1979;30:269–277. doi: 10.1146/annurev.me.30.020179.001413. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Davisson P. B., Gans P. J. Murine sarcoma viruses block corticosteroid-induced differentiation of bone marrow preadipocytes associated with long-term in vitro hemopoiesis. Virology. 1979 Jun;95(2):317–333. doi: 10.1016/0042-6822(79)90487-2. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Newburger P. E., Sakakeeny M. Phorbol myristate acetate stimulates macrophage differentiation and replication and alters granulopoiesis and leukemogenesis in long-term bone marrow cultures. Blood. 1980 Sep;56(3):368–379. [PubMed] [Google Scholar]
- HARAN-GHERA N., PULLAR P., FURTH J. Induction of thyrotropin-dependent thyroid tumors by thyrotropes. Endocrinology. 1960 May;66:694–701. doi: 10.1210/endo-66-5-694. [DOI] [PubMed] [Google Scholar]
- HUGGINS C., BERGENSTAL D. M. Inhibition of human mammary and prostatic cancers by adrenalectomy. Cancer Res. 1952 Feb;12(2):134–141. [PubMed] [Google Scholar]
- Huber B., Devinsky O., Gershon R. K., Cantor H. Cell-mediated immunity: delayed-type hypersensitivity and cytotoxic responses are mediated by different T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1534–1539. doi: 10.1084/jem.143.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. R., Burgess A. W. Molecular and biological properties of a macrophage colony-stimulating factor from mouse yolk sacs. J Cell Biol. 1978 Apr;77(1):35–47. doi: 10.1083/jcb.77.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc J. C., Cantor H. T cell-mediated immunity to oncornavirus-induced tumors. II. Ability of different T cell sets to prevent tumor growth in vivo. J Immunol. 1980 Feb;124(2):851–854. [PubMed] [Google Scholar]
- Lionetti F. J., Hunt S. M., Mattaliano R. J., Valeri C. R. In vitro studies of cryopreserved baboon granulocytes. Transfusion. 1978 Nov-Dec;18(6):685–692. doi: 10.1046/j.1537-2995.1978.18679077950.x. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro G. A., Maron E., Dray S. Antigen-secreting cells: enumeration of immunoglobulin-allotype-secreting cells in nonimmunized rabbits by means of hybrid-antibody-coated erythrocytes in a reverse hemolytic plaque assay. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1229–1233. doi: 10.1073/pnas.71.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G., Fresno M., Chessman A., Cantor H. Use of cloned populations of mouse lymphocytes to analyze cellular differentiation. Cell. 1981 Jan;23(1):19–28. doi: 10.1016/0092-8674(81)90266-x. [DOI] [PubMed] [Google Scholar]
- Nathan D. G., Chess L., Hillman D. G., Clarke B., Breard J., Merler E., Housman D. E. Human erythroid burst-forming unit: T-cell requirement for proliferation in vitro. J Exp Med. 1978 Feb 1;147(2):324–339. doi: 10.1084/jem.147.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintáns J., Lefkovits I. Precursor cells specific to sheep red cells in nude mice. Estimation of frequency in the microculture system. Eur J Immunol. 1973 Jul;3(7):392–397. doi: 10.1002/eji.1830030704. [DOI] [PubMed] [Google Scholar]
- Shaw J., Caplan B., Paetkau V., Pilarski L. M., Delovitch T. L., McKenzie I. F. Cellular origins of co-stimulator (IL2) and its activity in cytotoxic T lymphocyte responses. J Immunol. 1980 May;124(5):2231–2239. [PubMed] [Google Scholar]
- Stanley E. R. Colony-stimulating factor (CSF) radioimmunoassay: detection of a CSF subclass stimulating macrophage production. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2969–2973. doi: 10.1073/pnas.76.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Strelkauskas A. J., Eby W. C., Molinaro G. A., Dray S. Assessment of a B-cell function in chronic lymphocytic leukemia. Clin Immunol Immunopathol. 1976 Nov;6(3):334–340. doi: 10.1016/0090-1229(76)90086-6. [DOI] [PubMed] [Google Scholar]
- Wagner H., Röllinghoff M. T-T-cell interactions during the vitro cytotoxic allograft responses. I. Soluble products from activated Lyl+ T cells trigger autonomously antigen-primed Ly23+ T cells to cell proliferation and cytolytic activity. J Exp Med. 1978 Dec 1;148(6):1523–1538. doi: 10.1084/jem.148.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Gillis S., Marbrook J., Mochizuki D., Smith K. A. Biochemical and biological characterization of lymphocyte regulatory molecules. I. Purification of a class of murine lymphokines. J Exp Med. 1979 Oct 1;150(4):849–861. doi: 10.1084/jem.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]


