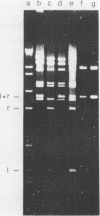
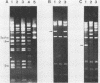
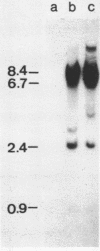
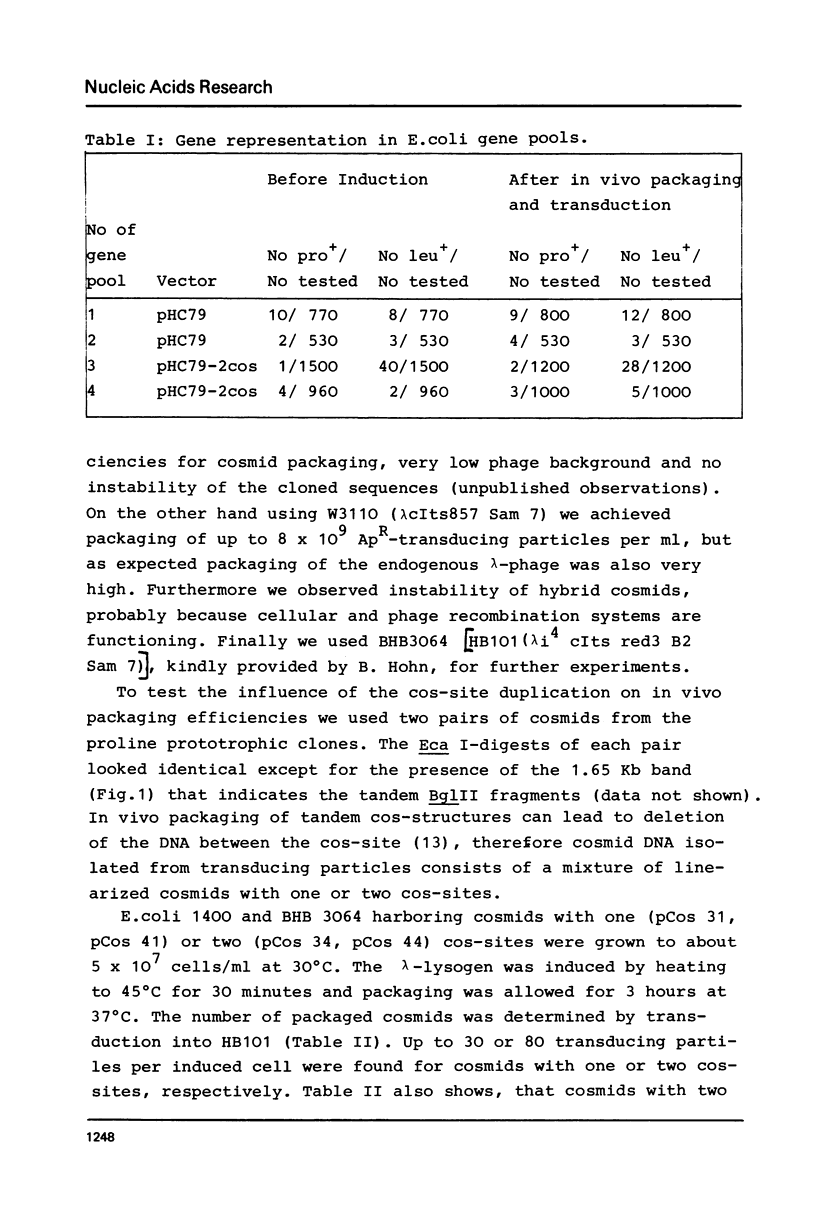
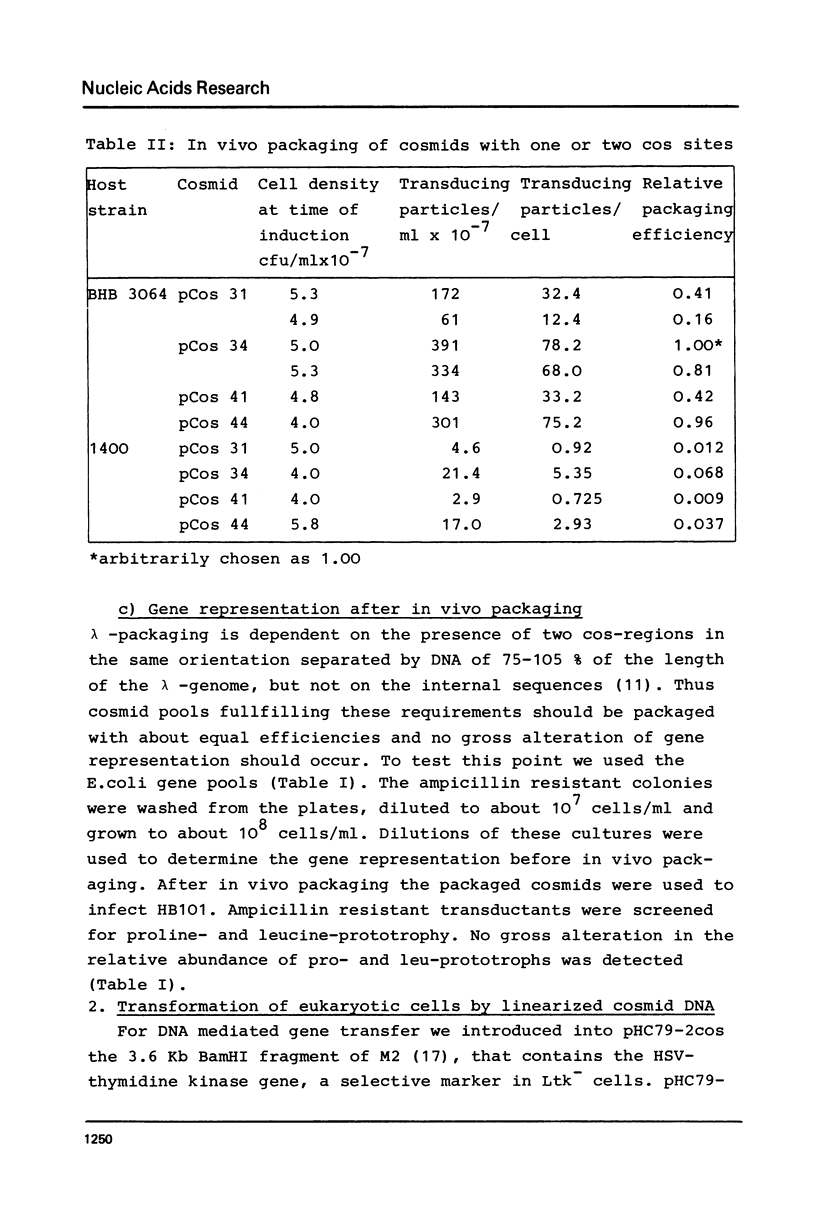
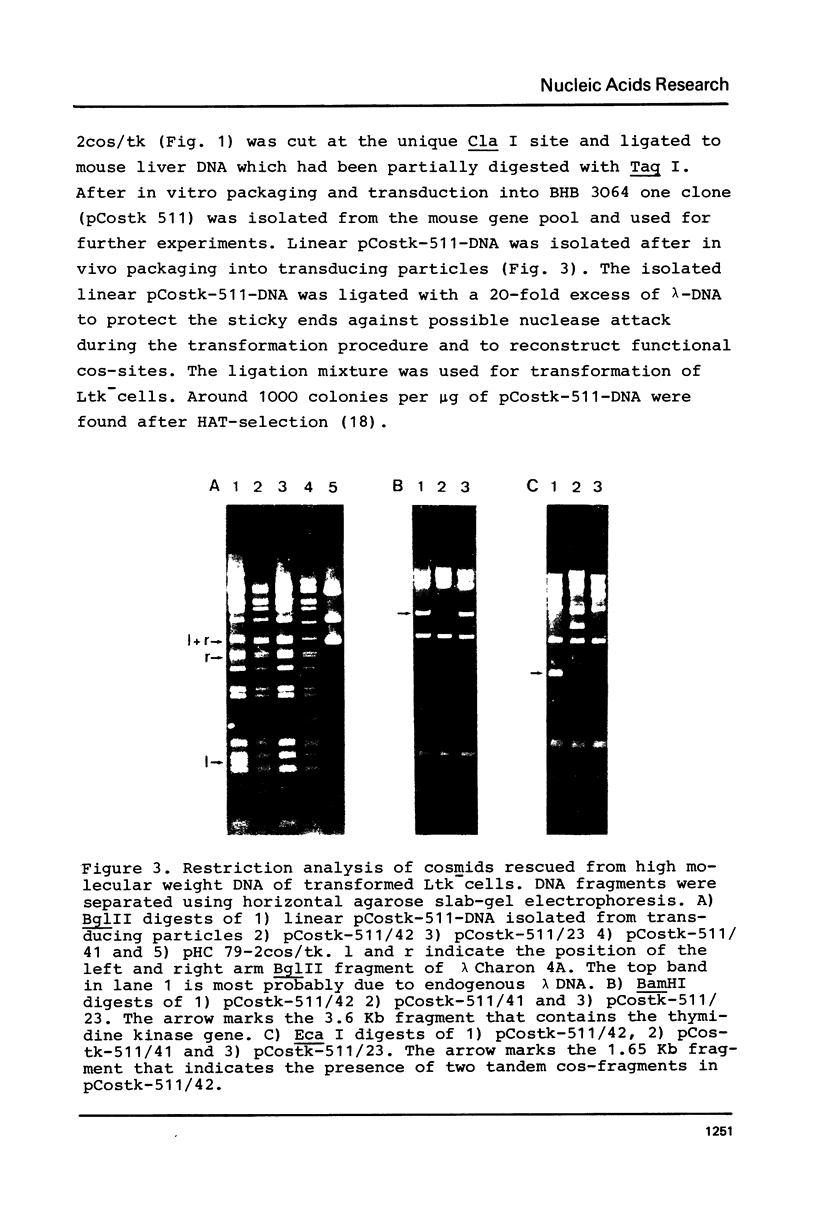
Abstract
Successful shuttling of cloned DNA in eukaryotic cells should allow isolation of expressed genes. We tested the utility of cosmids for moving DNA into and out of eukaryotic cells. The unique cleavage of DNA at the cos site by the terminase function of lambda was exploited to maintain the linkage between the vector and inserted gene sequences, a prerequisite for successful rescue of the transforming DNA from high molecular weight DNA of the eukaryotic transformant. A cosmid recombinant containing the HSV thymidine kinase gene and a lambda recombinant containing the chicken thymidine kinase gene were used to test the feasability of this method. It was found that these recombinants can be rescued with high efficiency from DNA of HAT-resistant cells.
Full text
PDF



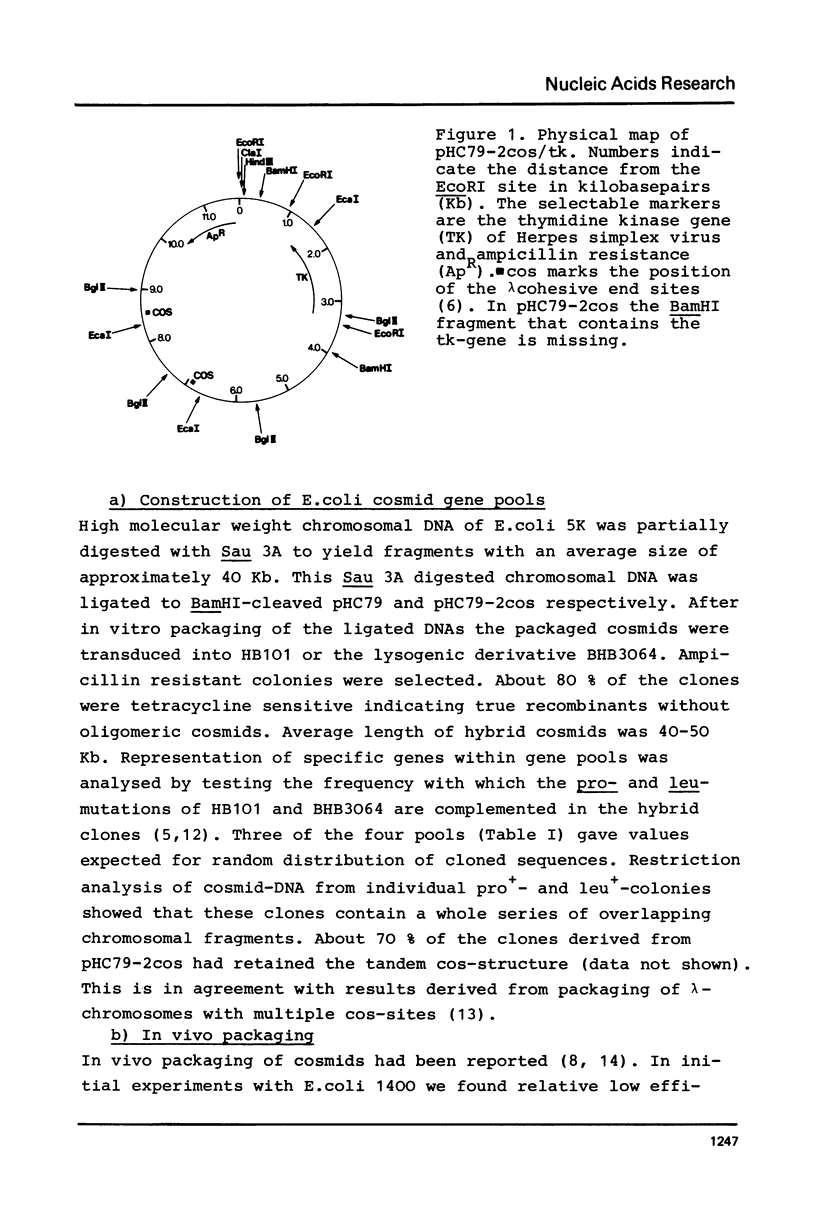






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cami B., Kourilsky P. Screening of cloned recombinant DNA in bacteria by in situ colony hybridization. Nucleic Acids Res. 1978 Jul;5(7):2381–2390. doi: 10.1093/nar/5.7.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Collins J., Brüning H. J. Plasmids useable as gene-cloning vectors in an in vitro packaging by coliphage lambda: "cosmids". Gene. 1978 Oct;4(2):85–107. doi: 10.1016/0378-1119(78)90023-9. [DOI] [PubMed] [Google Scholar]
- Feiss M., Bublitz A. Polarized packaging of bacteriophage lambda chromosomes. J Mol Biol. 1975 Jun 5;94(4):583–594. doi: 10.1016/0022-2836(75)90323-x. [DOI] [PubMed] [Google Scholar]
- Feiss M., Margulies T. On maturation of the bacteriophage lambda chromosome. Mol Gen Genet. 1973 Dec 31;127(4):285–295. doi: 10.1007/BF00267099. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Lane D., Lipsich L., Wigler M., Botchan M. Characteristics of an SV40-plasmid recombinant and its movement into and out of the genome of a murine cell. Cell. 1980 Aug;21(1):127–139. doi: 10.1016/0092-8674(80)90120-8. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Lindenmaier W., Nguyen-Huu M. C., Lurz R., Stratmann M., Blin N., Wurtz T., Hauser H. J., Sippel A. E., Schütz G. Arrangement of coding and intervening sequences of chicken lysozyme gene. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6196–6200. doi: 10.1073/pnas.76.12.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Lipsich L., Wigler M. Isolation of the chicken thymidine kinase gene by plasmid rescue. Nature. 1980 May 22;285(5762):207–210. doi: 10.1038/285207a0. [DOI] [PubMed] [Google Scholar]
- Scangos G., Ruddle F. H. Mechanisms and applications of DNA-mediated gene transfer in mammalian cells - a review. Gene. 1981 Jun-Jul;14(1-2):1–10. doi: 10.1016/0378-1119(81)90143-8. [DOI] [PubMed] [Google Scholar]
- Stiles J. I., Szostak J. W., Young A. T., Wu R., Consaul S., Sherman F. DNA sequence of a mutation in the leader region of the yeast iso-1-cytochrome c mRNA. Cell. 1981 Jul;25(1):277–284. doi: 10.1016/0092-8674(81)90253-1. [DOI] [PubMed] [Google Scholar]
- Umene K., Shimada K., Takagi Y. Packaging of ColE1 DNA having a lambda phage cohesive end site. Mol Gen Genet. 1978 Feb 7;159(1):39–45. doi: 10.1007/BF00401746. [DOI] [PubMed] [Google Scholar]
- Vollenweider H. J., Fiandt M., Rosenvold E. C., Szybalski W. Packaging of plasmid DNA containing the cohesive ends of coliphage lambda. Gene. 1980 Apr;9(1-2):171–174. doi: 10.1016/0378-1119(80)90174-2. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Boll W., Mantei N., Lonsdale D., Weissmann C. Hybrid plasmids containing an active thymidine kinase gene of Herpes simplex virus 1. Nucleic Acids Res. 1979 Oct 25;7(4):859–877. doi: 10.1093/nar/7.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Daniels D. L., Schroeder J. L., Williams B. G., Denniston-Thompson K., Moore D. D., Blattner F. R. Restriction maps for twenty-one Charon vector phages. J Virol. 1980 Jan;33(1):401–410. doi: 10.1128/jvi.33.1.401-410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]