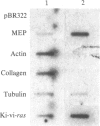
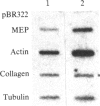
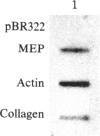
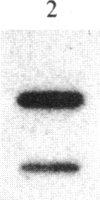
Abstract
Treatment of mouse NIH 3T3 cells with the phorbol ester tumor promoter, phorbol 12-myristate 13-acetate, results in altered transcription of several genes as measured in nuclear run-off experiments. The first set of genes, whose altered transcription occurs rapidly in the absence of protein synthesis, is typified by induction of c-myc and c-fos and decreased transcription of alpha 2 type I procollagen. This work demonstrates the existence of a second class of genes whose rapidly increased transcription requires prior protein synthesis, which is represented by the gene encoding a secreted lysosomal protein, MEP. Similar induction of MEP RNA is seen after treatment with platelet-derived growth factor or transformation with Kirsten sarcoma virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belin D., Godeau F., Vassalli J. D. Tumor promoter PMA stimulates the synthesis and secretion of mouse pro-urokinase in MSV-transformed 3T3 cells: this is mediated by an increase in urokinase mRNA content. EMBO J. 1984 Aug;3(8):1901–1906. doi: 10.1002/j.1460-2075.1984.tb02065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. Activation of a Qa/Tla class I major histocompatibility antigen gene is a general feature of oncogenesis in the mouse. Nature. 1983 Dec 22;306(5945):756–760. doi: 10.1038/306756a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. J., Hua L., Liau G., Gal S., Graham D. E., Sobel M., Gottesman M. M. Malignant transformation and tumor promoter treatment increase levels of a transcript for a secreted glycoprotein. Mol Cell Biol. 1985 Mar;5(3):466–473. doi: 10.1128/mcb.5.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraiuolo R., Stoppelli M. P., Verde P., Bullock S., Lazzaro P., Blasi F., Pietropaolo T. C. Transcriptional induction of urokinase in cultured human kidney carcinoma cells by tetradecanoyl-phorbol-acetate. J Cell Physiol. 1984 Nov;121(2):368–374. doi: 10.1002/jcp.1041210214. [DOI] [PubMed] [Google Scholar]
- Frick K. K., Doherty P. J., Gottesman M. M., Scher C. D. Regulation of the transcript for a lysosomal protein: evidence for a gene program modified by platelet-derived growth factor. Mol Cell Biol. 1985 Oct;5(10):2582–2589. doi: 10.1128/mcb.5.10.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal S., Willingham M. C., Gottesman M. M. Processing and lysosomal localization of a glycoprotein whose secretion is transformation stimulated. J Cell Biol. 1985 Feb;100(2):535–544. doi: 10.1083/jcb.100.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Sobel M. E. Tumor promoters and Kirsten sarcoma virus increase synthesis of a secreted glycoprotein by regulating levels of translatable mRNA. Cell. 1980 Feb;19(2):449–455. doi: 10.1016/0092-8674(80)90519-x. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M. Transformation-dependent secretion of a low molecular weight protein by murine fibroblasts. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2767–2771. doi: 10.1073/pnas.75.6.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Todaro G. J., Goldberg B. Collagen synthesis in fibroblasts transformed by oncogenic viruses. Nature. 1966 Feb 26;209(5026):916–917. doi: 10.1038/209916a0. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. L., Dudley L., Dobner P. R., Lewis S. A., Cowan N. J. Identification of two human beta-tubulin isotypes. Mol Cell Biol. 1983 May;3(5):854–862. doi: 10.1128/mcb.3.5.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Laszlo A., Radke K., Chin S., Bissell M. J. Tumor promoters alter gene expression and protein phosphorylation in avian cells in culture. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6241–6245. doi: 10.1073/pnas.78.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys E. J., Crouse G. F., Kellems R. E. Dihydrofolate reductase gene expression in cultured mouse cells is regulated by transcript stabilization in the nucleus. J Cell Biol. 1984 Jul;99(1 Pt 1):180–187. doi: 10.1083/jcb.99.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau G., Yamada Y., de Crombrugghe B. Coordinate regulation of the levels of type III and type I collagen mRNA in most but not all mouse fibroblasts. J Biol Chem. 1985 Jan 10;260(1):531–536. [PubMed] [Google Scholar]
- Murphy D., Brickell P. M., Latchman D. S., Willison K., Rigby P. W. Transcripts regulated during normal embryonic development and oncogenic transformation share a repetitive element. Cell. 1983 Dec;35(3 Pt 2):865–871. doi: 10.1016/0092-8674(83)90119-8. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky B., Prather W. B. Increased collagen synthesis in Kirsten sarcoma virus-transformed BALB 3T3 cells grown in the presence of dibutyryl cyclic AMP. Cell. 1974 Nov;3(3):291–299. doi: 10.1016/0092-8674(74)90144-5. [DOI] [PubMed] [Google Scholar]
- Sahagian G. G., Gottesman M. M. The predominant secreted protein of transformed murine fibroblasts carries the lysosomal mannose 6-phosphate recognition marker. J Biol Chem. 1982 Sep 25;257(18):11145–11150. [PubMed] [Google Scholar]
- Scher C. D., Dick R. L., Whipple A. P., Locatell K. L. Identification of a BALB/c-3T3 cell protein modulated by platelet-derived growth factor. Mol Cell Biol. 1983 Jan;3(1):70–81. doi: 10.1128/mcb.3.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Yamada Y., de Crombrugghe B. DNA sequence comparison of the regulatory signals at the 5' end of the mouse and chick alpha 2 type I collagen genes. J Biol Chem. 1984 Jun 25;259(12):7411–7415. [PubMed] [Google Scholar]
- Schutzbank T., Robinson R., Oren M., Levine A. J. SV40 large tumor antigen can regulate some cellular transcripts in a positive fashion. Cell. 1982 Sep;30(2):481–490. doi: 10.1016/0092-8674(82)90245-8. [DOI] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Sobel M. E., Dion L. D., Vuust J., Colburn N. H. Tumor-promoting phorbol esters inhibit procollagen synthesis at a pretranslational level in JB-6 mouse epidermal cells. Mol Cell Biol. 1983 Aug;3(8):1527–1532. doi: 10.1128/mcb.3.8.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D. The molecular biology of platelet-derived growth factor. Cell. 1983 Jul;33(3):653–655. doi: 10.1016/0092-8674(83)90008-9. [DOI] [PubMed] [Google Scholar]
- Tyagi J. S., Hirano H., Merlino G. T., Pastan I. Transcriptional control of the fibronectin gene in chick embryo fibroblasts transformed by Rous sarcoma virus. J Biol Chem. 1983 May 10;258(9):5787–5793. [PubMed] [Google Scholar]
- Verde P., Stoppelli M. P., Galeffi P., Di Nocera P., Blasi F. Identification and primary sequence of an unspliced human urokinase poly(A)+ RNA. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4727–4731. doi: 10.1073/pnas.81.15.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Weston J. A. Isolation of a major cell surface glycoprotein from fibroblasts. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3492–3496. doi: 10.1073/pnas.71.9.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]