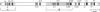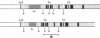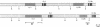Abstract
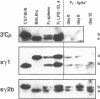
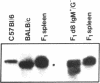
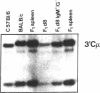
Mouse B lymphocytes can be activated polyclonally by bacterial lipopolysaccharide (LPS) to secrete Ig and perform Ig class switch. In the presence of the T-cell lymphokine B-cell differentiation factor, the frequency of IgG1-secreting cells is drastically enhanced. We show here that IgG1-secreting B cells isolated from such cultures have undergone a similar DNA rearrangement of the switch regions (S mu, S gamma 1) of the Ig heavy chain constant region genes C mu and C gamma 1 on both active and inactive IgH loci. This result argues against a stochastic model of class switch recombination and suggests programmed class-specific switch recombination in the case of the switch to IgG1. In accord with this notion, cells expressing IgM but not IgG on the surface have not deleted or rearranged C mu or S gamma 1 on either chromosome.
Full text
PDF

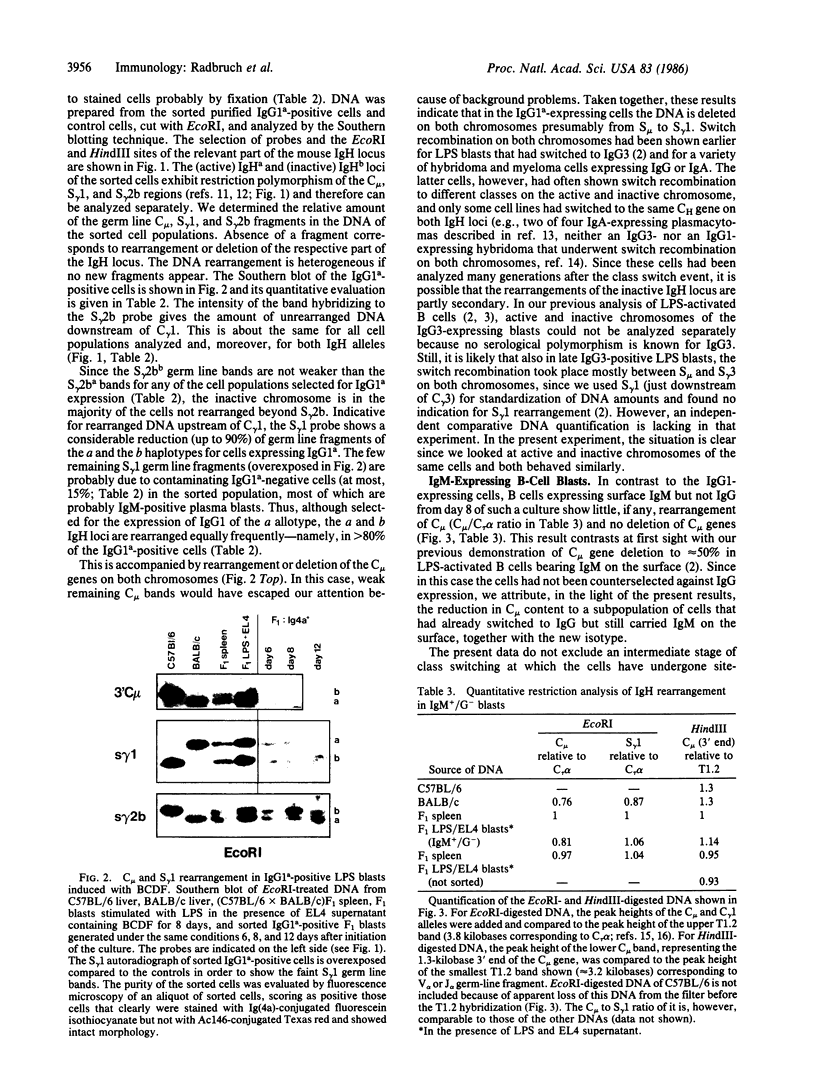

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Cooper D., Perry R. P. Rearrangement of immunoglobulin heavy chain genes during B-lymphocyte development as revealed by studies of mouse plasmacytoma cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1422–1426. doi: 10.1073/pnas.77.3.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Forni L. Intraclonal diversification in immunoglobulin isotype secretion: an analysis of switch probabilities. EMBO J. 1982;1(10):1251–1257. doi: 10.1002/j.1460-2075.1982.tb00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Kim S. K., Hood L. E. DNA sequences mediating class switching in alpha-immunoglobulins. Science. 1980 Sep 19;209(4463):1360–1365. doi: 10.1126/science.6774415. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Bannwarth W., Taylor B. A., Steinmetz M. The gene encoding the T-cell receptor alpha-chain maps close to the Np-2 locus on mouse chromosome 14. Nature. 1985 Mar 21;314(6008):271–273. doi: 10.1038/314271a0. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Heck J. A., Strober W. T-cell regulation of murine IgA synthesis. J Exp Med. 1979 Mar 1;149(3):632–643. doi: 10.1084/jem.149.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A. C., Diamond D. J., Tanigawa G., Heilig J. S., Folsom V., Saito H., Tonegawa S. Unusual organization and diversity of T-cell receptor alpha-chain genes. 1985 Aug 29-Sep 4Nature. 316(6031):828–832. doi: 10.1038/316828a0. [DOI] [PubMed] [Google Scholar]
- Hirashima M., Yodoi J., Ishizaka K. Regulatory role of IgE-binding factors from rat T lymphocytes. V. formation of IgE-potentiating factor by T lymphocytes from rats treated with Bordetella pertussis vaccine. J Immunol. 1981 Mar;126(3):838–842. [PubMed] [Google Scholar]
- Huang C. M., Parsons M., Oi V. T., Huang H. J., Herzenberg L. A. Genetic characterization of mouse immunoglobulin allotypic determinants (allotopes) defined by monoclonal antibodies. Immunogenetics. 1983;18(4):311–321. doi: 10.1007/BF00372464. [DOI] [PubMed] [Google Scholar]
- Hurwitz J. L., Coleclough C., Cebra J. J. CH gene rearrangements in IgM-bearing B cells and in the normal splenic DNA component of hybridomas making different isotypes of antibody. Cell. 1980 Nov;22(2 Pt 2):349–359. doi: 10.1016/0092-8674(80)90345-1. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Vitetta E. S., Krammer P. H. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982 Mar 1;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi H., Saltzman L. E., Strober W. Characteristics and regulatory function of murine con A-induced, cloned T cells obtained from Peyer's patches and spleen: mechanisms regulating isotype-specific immunoglobulin production by Peyer's patch B cells. J Immunol. 1982 Aug;129(2):475–483. [PubMed] [Google Scholar]
- Kishimoto T., Ishizaka K. Regulation of antibody response in vitro. VII. Enhancing soluble factors for IgG and IgE antibody response. J Immunol. 1973 Oct;111(4):1194–1205. [PubMed] [Google Scholar]
- Klein S., Sablitzky F., Radbruch A. Deletion of the IgH enhancer does not reduce immunoglobulin heavy chain production of a hybridoma IgD class switch variant. EMBO J. 1984 Nov;3(11):2473–2476. doi: 10.1002/j.1460-2075.1984.tb02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton J. E., Vitetta E. S., Uhr J. W., Krammer P. H. Clonal analysis of B cells induced to secrete IgG by T cell-derived lymphokine(s). J Exp Med. 1984 Dec 1;160(6):1850–1863. doi: 10.1084/jem.160.6.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B., Banerji J., Penncavage N. A., Lang R., Arnheim N. 5' flanking region of immunoglobulin heavy chain constant region genes displays length heterogeneity in germlines of inbred mouse strains. Cell. 1980 Nov;22(1 Pt 1):187–196. doi: 10.1016/0092-8674(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Mowatt M., Dery C., Dunnick W. Unique sequences are interspersed among tandemly repeated elements in the murine gamma 1 switch segment. Nucleic Acids Res. 1985 Jan 11;13(1):225–237. doi: 10.1093/nar/13.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Kühn R., Goldmann W., Tesch H., Smith F. I., Radbruch A., Rajewsky K. Signal requirements for growth and differentiation of activated murine B lymphocytes. J Immunol. 1985 Aug;135(2):1213–1219. [PubMed] [Google Scholar]
- Perlmutter A. P., Gilbert W. Antibodies of the secondary response can be expressed without switch recombination in normal mouse B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7189–7193. doi: 10.1073/pnas.81.22.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbruch A., Sablitzky F. Deletion of Cmu genes in mouse B lymphocytes upon stimulation with LPS. EMBO J. 1983;2(11):1929–1935. doi: 10.1002/j.1460-2075.1983.tb01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Takahashi N., Yaoita Y., Honjo T. Organization of the constant-region gene family of the mouse immunoglobulin heavy chain. Cell. 1982 Mar;28(3):499–506. doi: 10.1016/0092-8674(82)90204-5. [DOI] [PubMed] [Google Scholar]
- Weichel W., Liesegang B., Gehrke K., Göttlinger C., Holtkamp B., Radbruch A., Stackhouse T. K., Rajewsky K. Inexpensive upgrading of a FACS I and isolation of rare somatic variants by double-fluorescence sorting. Cytometry. 1985 Mar;6(2):116–123. doi: 10.1002/cyto.990060206. [DOI] [PubMed] [Google Scholar]
- Winoto A., Mjolsness S., Hood L. Genomic organization of the genes encoding mouse T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):832–836. doi: 10.1038/316832a0. [DOI] [PubMed] [Google Scholar]
- Yaoita Y., Honjo T. Deletion of immunoglobulin heavy chain genes from expressed allelic chromosome. Nature. 1980 Aug 28;286(5776):850–853. doi: 10.1038/286850a0. [DOI] [PubMed] [Google Scholar]