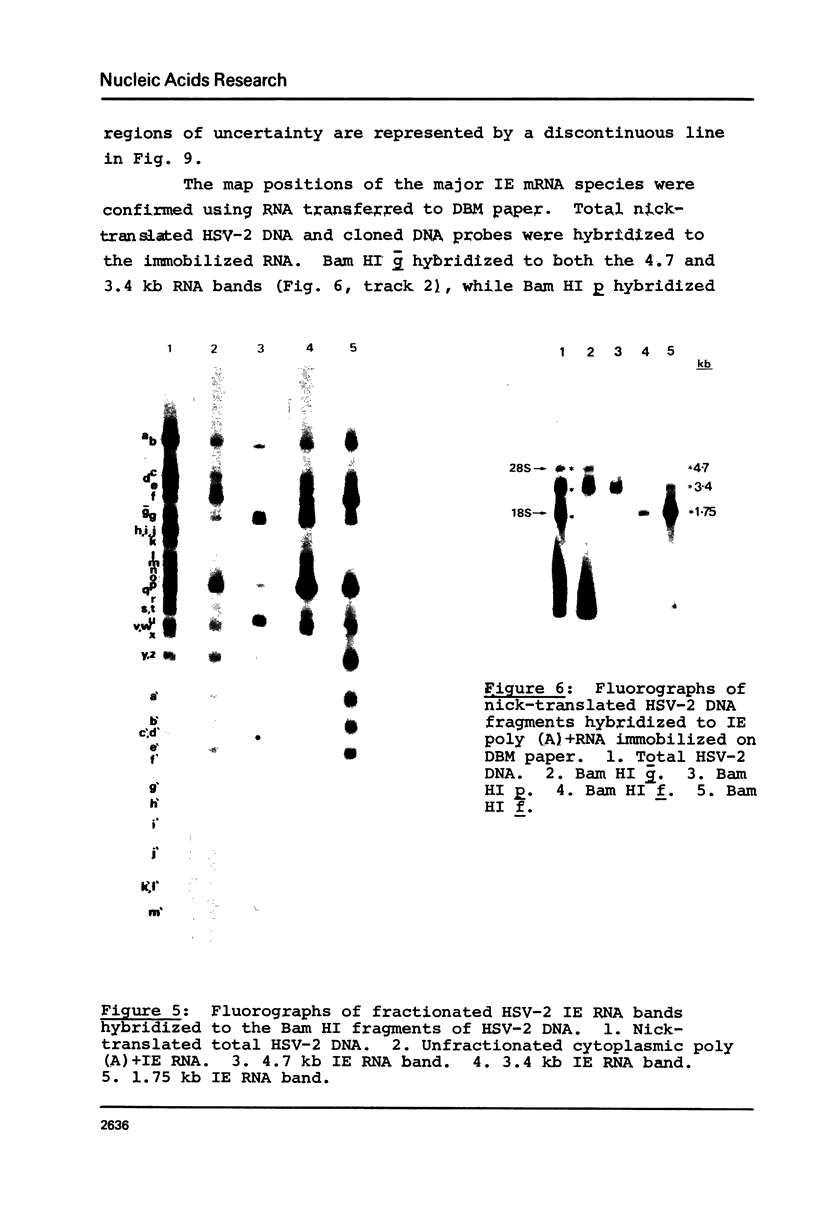
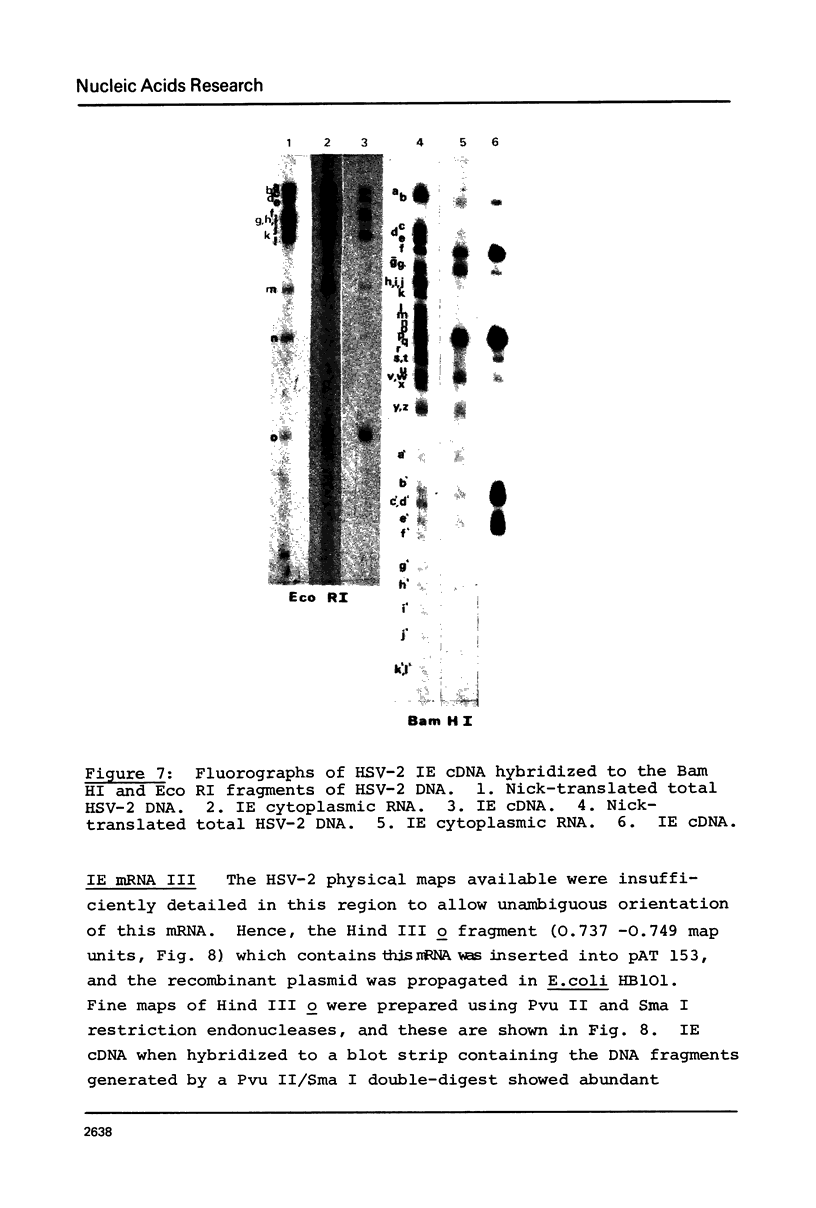
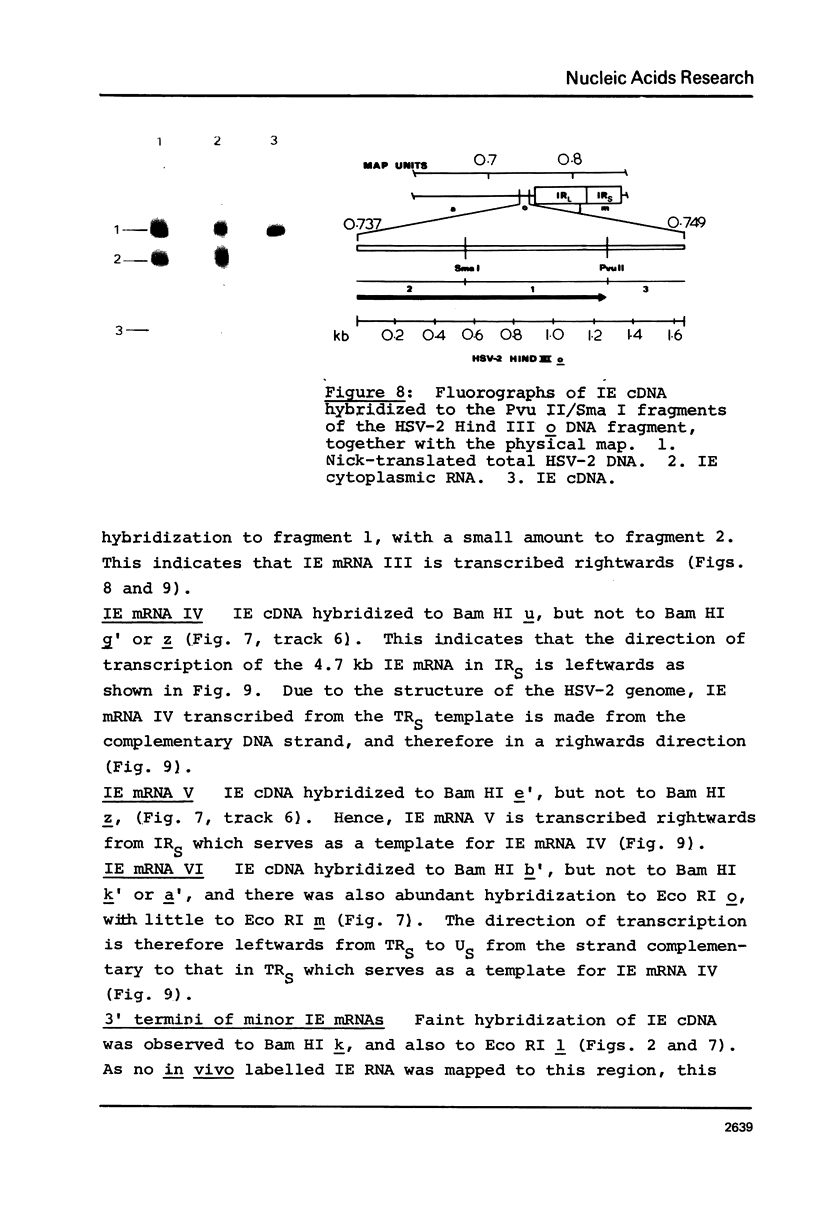
Abstract
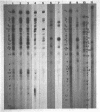
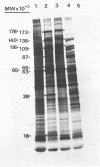
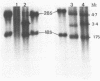
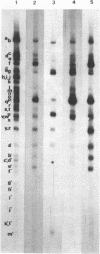
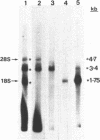
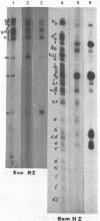
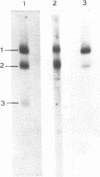
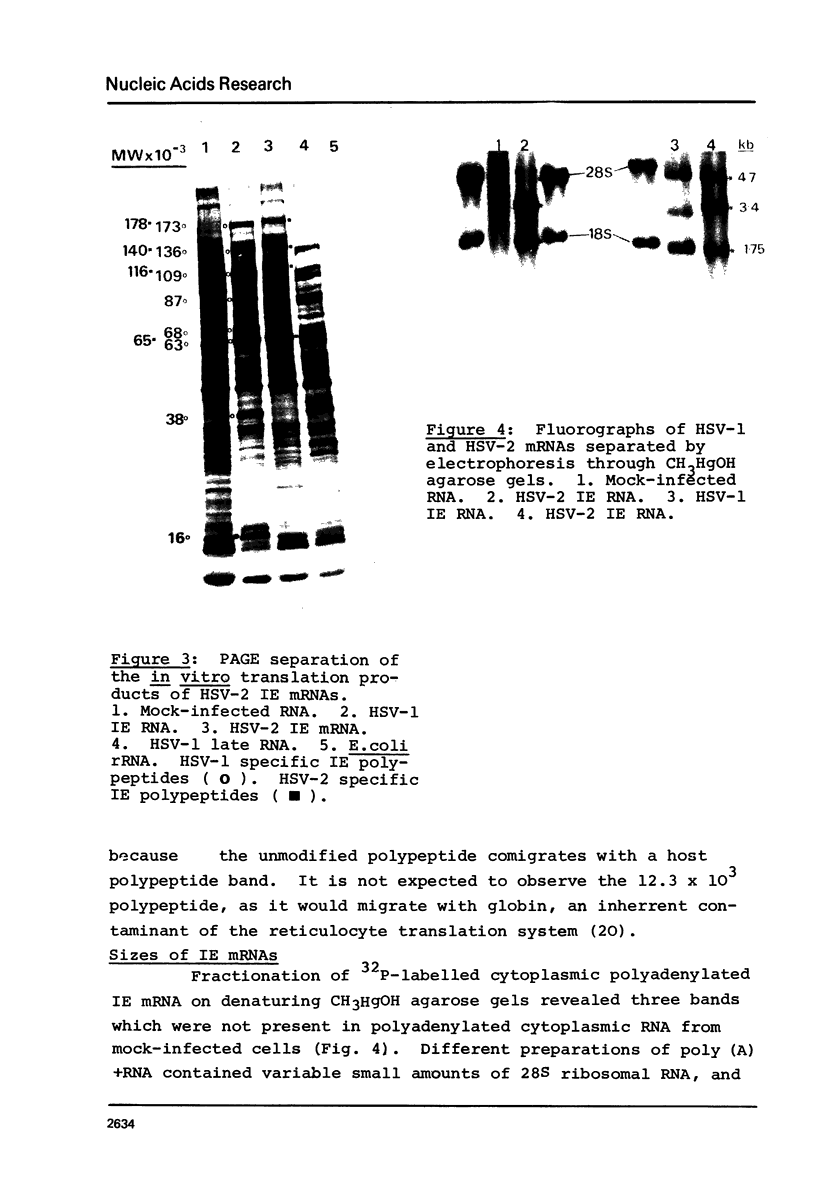
Nuclear and cytoplasmic virus RNAs, synthesized in cells infected with herpes simplex virus type 2 at early and late times post-infection, and in the continuous presence of the protein synthesis inhibitor cycloheximide (immediate early), have been analyzed by blot hybridization to virus DNA fragments generated by Bam HI and Eco RI restriction endonucleases. Polyadenylated immediate early mRNAs were separated on denaturing gels containing CH3HgOH giving three virus-specific mRNA bands of estimated sizes 4.7, 3.4 and 1.75 kb, and these have been mapped to five discrete regions of the genome. The polypeptides produced by in vitro translation of the HSV-2 immediate early mRNA's have been identified. Orientations of immediate early mRNA's on the virus genome have been determined by mapping cDNAs complementary to the 3'termini of the mRNAs.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker Y., Dym H., Sarov I. Herpes simplex virus DNA. Virology. 1968 Oct;36(2):184–192. doi: 10.1016/0042-6822(68)90135-9. [DOI] [PubMed] [Google Scholar]
- Bodemer W. W., Bodemer M. Partial characterization of herpes simplex virus type 2 (HSV-2)-specific poly(A)+ RNA by hybridization to EcoRI-generated HSV-2 DNA fragments. Virology. 1979 Jan 30;92(2):507–517. doi: 10.1016/0042-6822(79)90153-3. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Clements J. B., Cortini R., Wilkie N. M. Analysis of herpesvirus DNA substructure by means of restriction endonucleases. J Gen Virol. 1976 Feb;30(2):243–256. doi: 10.1099/0022-1317-30-2-243. [DOI] [PubMed] [Google Scholar]
- Clements J. B., McLauchlan J., McGeoch D. J. Orientation of herpes simplex virus type 1 immediate early mRNA's. Nucleic Acids Res. 1979 Sep 11;7(1):77–91. doi: 10.1093/nar/7.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. B., Watson R. J., Wilkie N. M. Temporal regulation of herpes simplex virus type 1 transcription: location of transcripts on the viral genome. Cell. 1977 Sep;12(1):275–285. doi: 10.1016/0092-8674(77)90205-7. [DOI] [PubMed] [Google Scholar]
- Cortini R., Wilkie N. M. Physical maps for HSV type 2 DNA with five restriction endonucleases. J Gen Virol. 1978 May;39(2):259–280. doi: 10.1099/0022-1317-39-2-259. [DOI] [PubMed] [Google Scholar]
- Delius H., Clements J. B. A partial denaturation map of herpes simplex virus type 1 DNA: evidence for inversions of the unique DNA regions. J Gen Virol. 1976 Oct;33(1):125–133. doi: 10.1099/0022-1317-33-1-125. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster embryo cells after exposure to inactivated herpes simplex virus type 1. J Virol. 1973 Aug;12(2):209–217. doi: 10.1128/jvi.12.2.209-217.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza J., Benyesh-Melnick B., Schaffer P. A. Intertypic complementation and recombination between temperature-sensitive mutants of herpes simplex virus types 1 and 2. Virology. 1976 Apr;70(2):372–384. doi: 10.1016/0042-6822(76)90279-8. [DOI] [PubMed] [Google Scholar]
- Feldman L., Rixon F. J., Jean J. H., Ben-Porat T., Kaplan A. S. Transcription of the genome of pseudorabies virus (A herpesvirus) is strictly controlled. Virology. 1979 Sep;97(2):316–327. doi: 10.1016/0042-6822(79)90343-x. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus: controls of transcription and of RNA abundance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2654–2658. doi: 10.1073/pnas.69.9.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Silverstein S., Cassai E., Roizman B. RNA synthesis in cells infected with herpes simplex virus. VII. Control of transcription and of transcript abundancies of unique and common sequences of herpes simplex virus 1 and 2. J Virol. 1973 Jun;11(6):886–892. doi: 10.1128/jvi.11.6.886-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A. N., Ray M., Megaw J., Brown R., Nahmias A. J. Common antigens of Herpex simplex virus 2, associated hamster tumors, and human cervical cancer. Proc Soc Exp Biol Med. 1976 Jul;152(3):343–347. doi: 10.3181/00379727-152-39393. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Macnab J. C., Perbal B., Clements J. B. Virus specified enzyme activity and RNA species in herpes simplex virus type 1 transformed mouse cells. J Gen Virol. 1976 Sep;32(3):493–508. doi: 10.1099/0022-1317-32-3-493. [DOI] [PubMed] [Google Scholar]
- Jones K. W., Fenoglio C. M., Shevchuk-Chaban M., Maitland N. J., McDougall J. K. Detection of herpes simplex virus type 2 mRNA in human cervical biopsies by in situ cytological hybridization. IARC Sci Publ. 1978;(24 Pt 2):917–925. [PubMed] [Google Scholar]
- Jones P. C., Roizman B. Regulation of herpesvirus macromolecular synthesis. VIII. The transcription program consists of three phases during which both extent of transcription and accumulation of RNA in the cytoplasm are regulated. J Virol. 1979 Aug;31(2):299–314. doi: 10.1128/jvi.31.2.299-314.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E., Hoyer B., Bachenheimer S., Roizman B. Genetic relatedness of type 1 and type 2 herpes simplex viruses. J Virol. 1972 May;9(5):738–745. doi: 10.1128/jvi.9.5.738-745.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M., Roizman B. Regulation of herpesvirus macromolecular synthesis: nuclear retention of nontranslated viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4322–4326. doi: 10.1073/pnas.71.11.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Lindberg U. Characterization of messenger ribonucleoprotein and messenger RNA from KB cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):681–685. doi: 10.1073/pnas.69.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H. O., Biswal N., Benyesh-Melnick M. Studies on the relatedness of herpesviruses through DNA-DNA hybridization. Virology. 1972 Jul;49(1):95–101. doi: 10.1016/s0042-6822(72)80010-2. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Buchman T. G., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus DNA. IX. Apparent exclusion of some parental DNA arrangements in the generation of intertypic (HSV-1 X HSV-2) recombinants. J Virol. 1977 Oct;24(1):231–248. doi: 10.1128/jvi.24.1.231-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Naib Z. M., Josey W. E. Epidemiological studies relating genital herpetic infection to cervical carcinoma. Cancer Res. 1974 May;34(5):1111–1117. [PubMed] [Google Scholar]
- Oakes J. E., Hyman R. W., Rapp F. Genome location of polyadenylated transcripts of herpes simplex virus type 1 and type 2 DNA. Virology. 1976 Nov;75(1):145–154. doi: 10.1016/0042-6822(76)90013-1. [DOI] [PubMed] [Google Scholar]
- Preston C. M. The cell-free synthesis of herpesvirus-induced polypeptides. Virology. 1977 May 1;78(1):349–353. doi: 10.1016/0042-6822(77)90109-x. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Davison A. J., Marsden H. S., Timbury M. C., Subak-Sharpe J. H., Wilkie N. M. Recombinants between herpes simplex virus types 1 and 2: analyses of genome structures and expression of immediate early polypeptides. J Virol. 1978 Nov;28(2):499–517. doi: 10.1128/jvi.28.2.499-517.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. Physical mapping of temperature-sensitive mutations of herpes simplex virus type 1 by intertypic marker rescue. Virology. 1978 Oct 1;90(1):1–11. doi: 10.1016/0042-6822(78)90327-6. [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe J. H., Brown S. M., Ritchie D. A., Timbury M. C., Macnab J. C., Marsden H. S., Hay J. Genetic and biochemical studies with herpesvirus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):717–730. doi: 10.1101/sqb.1974.039.01.085. [DOI] [PubMed] [Google Scholar]
- Timbury M. C., Subak-Sharpe J. H. Genetic interactions between temperature-sensitive mutants of types 1 and 2 herpes simplex viruses. J Gen Virol. 1973 Mar;18(3):347–357. doi: 10.1099/0022-1317-18-3-347. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Summers W. C. Structure of the joint region and the termini of the DNA of herpes simplex virus type 1. J Virol. 1978 Aug;27(2):374–387. doi: 10.1128/jvi.27.2.374-387.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. A herpes simplex virus type 1 function continuously required for early and late virus RNA synthesis. Nature. 1980 May 29;285(5763):329–330. doi: 10.1038/285329a0. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. Characterization of transcription-deficient temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1978 Dec;91(2):364–379. doi: 10.1016/0042-6822(78)90384-7. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Macnab J. C., Subak-Sharpe J. H. The structure and biological properties of herpes simplex virus DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):657–666. doi: 10.1101/sqb.1974.039.01.079. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Davison A., Chartrand P., Stow N. D., Preston V. G., Timbury M. C. Recombination in herpes simplex virus: mapping of mutations and analysis of intertypic recombinants. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):827–840. doi: 10.1101/sqb.1979.043.01.089. [DOI] [PubMed] [Google Scholar]