Abstract
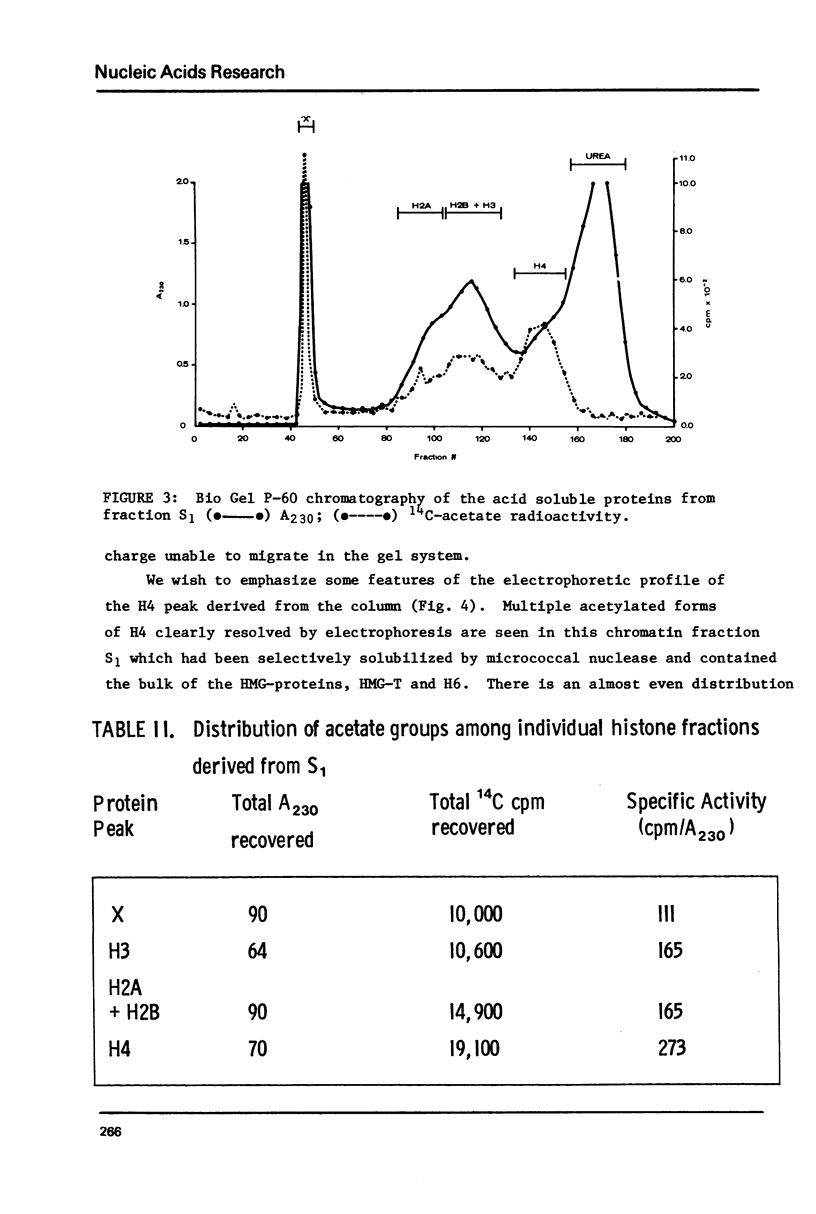
We have examined the distribution of acetylated histones derived from various trout testis chromatin fractions of different composition. Our results indicate that a chromatin fraction, preferentially solubilized by micrococcal nuclease, containing the bulk of the HMG proteins and similar to a fraction released from intact trout nuclei and previously shown to be enriched in transcribed DNA sequences also possesses high levels of multiacetylated species of H4. Histones 2A, 2B and 3 are also acetylated in this particular chromatin fraction. Monoacetylated species of the 4 inner nucleosomal histones appear to be characteristic of the nucleohistone portion of trout testis chromatin.
Full text
PDF






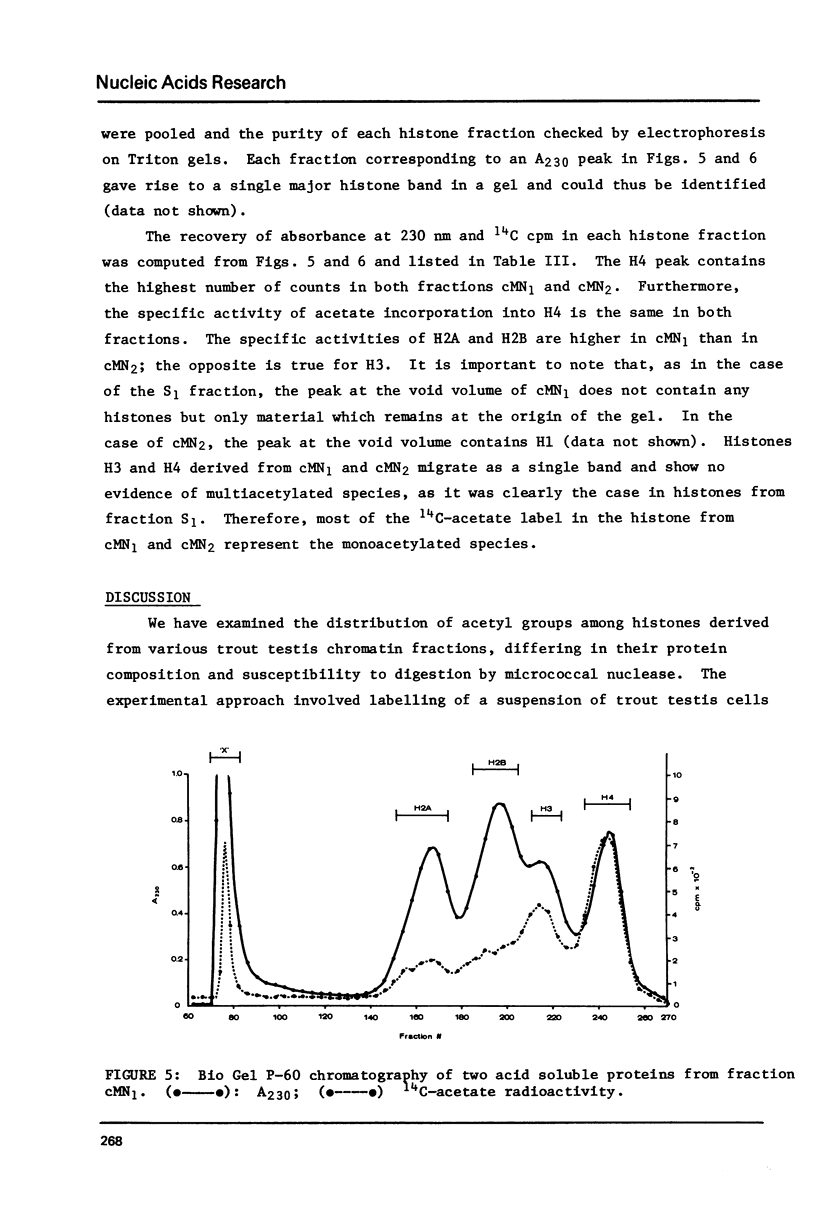
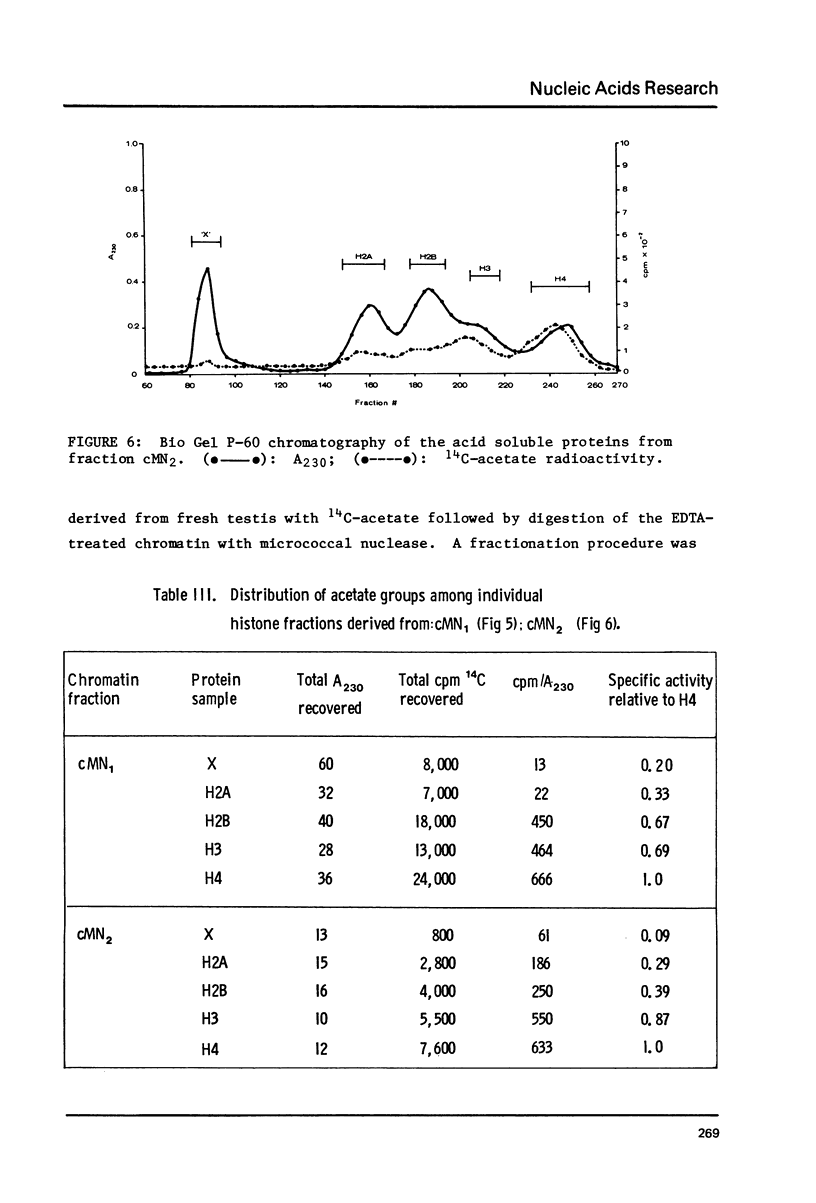





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellard M., Gannon F., Chambon P. Nucleosome structure III: the structure and transcriptional activity of the chromatin containing the ovalbumin and globin genes in chick oviduct nuclei. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):779–791. doi: 10.1101/sqb.1978.042.01.078. [DOI] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Davie J. R., Candido E. P. Acetylated histone H4 is preferentially associated with template-active chromatin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3574–3577. doi: 10.1073/pnas.75.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Smith E. L. Histones: structure and function. Annu Rev Biochem. 1971;40:279–314. doi: 10.1146/annurev.bi.40.070171.001431. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Pleger G. L., Keevert J. B., Johmann C. A. Studies on histone fraction F2A1 in macro- and micronuclei of Tetrahymena pyriformis. J Cell Biol. 1973 Jun;57(3):773–781. doi: 10.1083/jcb.57.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Wilson B., Gjerset R. A., McCarthy B. J. Acetylation and phosphorylation of Drosophila histones. Distribution of acetate and phosphate groups in fractionated chromatin. Biochim Biophys Acta. 1977 Mar 2;475(1):168–175. doi: 10.1016/0005-2787(77)90351-3. [DOI] [PubMed] [Google Scholar]
- Levy B., Dixon G. H. Renaturation kinetics of cDNA complementary to cytoplamic polyadenylated RNA from rainbow trout testis. Accessibility of transcribed genes to pancreatic DNase. Nucleic Acids Res. 1977 Apr;4(4):883–898. doi: 10.1093/nar/4.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B., Wong N. C., Watson D. C., Peters E. H., Dixon G. H. Structure and function of the low-salt extractable chromosomal proteins. Preferential association of trout testis proteins H6 and HMG-T with chromatin regions selectively sensitive to nucleases. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):793–801. doi: 10.1101/sqb.1978.042.01.079. [DOI] [PubMed] [Google Scholar]
- Libby P. R. Histone acetylation by cell-free preparations from rat uterus: in vitro stimulation by estradiol-17 beta. Biochem Biophys Res Commun. 1968 Apr 5;31(1):59–65. doi: 10.1016/0006-291x(68)90031-4. [DOI] [PubMed] [Google Scholar]
- Louie A. J., Candido E. P., Dixon G. H. Enzymatic modifications and their possible roles in regulating the binding of basic proteins to DNA and in controlling chromosomal structure. Cold Spring Harb Symp Quant Biol. 1974;38:803–819. doi: 10.1101/sqb.1974.038.01.084. [DOI] [PubMed] [Google Scholar]
- Marushige K., Dixon G. H. Developmental changes in chromosomal composition and template activity during spermatogenesis in trout testis. Dev Biol. 1969 Apr;19(4):397–414. doi: 10.1016/0012-1606(69)90050-5. [DOI] [PubMed] [Google Scholar]
- Marushige K., Marushige Y., Wong T. K. Complete displacement of somatic histones during transformation of spermatid chromatin: a model experiment. Biochemistry. 1976 May 18;15(10):2047–2053. doi: 10.1021/bi00655a004. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Jr, McCarty K. S. Two classes of histone acetylation in developing mouse mammary gland. J Biol Chem. 1970 Nov 10;245(21):5635–5642. [PubMed] [Google Scholar]
- Peter E., Candido M., Dixon G. H. Acetylation of trout testis histones in vivo. Site of the modification in histone IIb 1 . J Biol Chem. 1972 Jun 25;247(12):3868–3873. [PubMed] [Google Scholar]
- Pogo B. G., Allfrey V. G., Mirsky A. E. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc Natl Acad Sci U S A. 1966 Apr;55(4):805–812. doi: 10.1073/pnas.55.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Pogo A. O., Allfrey V. G., Mirsky A. E. Changing patterns of histone acetylation and RNA synthesis in regeneration of the liver. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1337–1344. doi: 10.1073/pnas.59.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Allfrey V. G. Processing of newly synthesized histone molecules. Science. 1975 Oct 10;190(4210):117–128. doi: 10.1126/science.1166303. [DOI] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. DNA associated with hyperacetylated histone is preferentially digested by DNase I. Nucleic Acids Res. 1978 Jun;5(6):1863–1876. doi: 10.1093/nar/5.6.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T. Structure of chromatin containing extensively acetylated H3 and H4. Cell. 1978 Apr;13(4):691–699. doi: 10.1016/0092-8674(78)90219-2. [DOI] [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Bradbury E. M., Allfrey V. G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A. 1978 May;75(5):2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. C., Peters E. H., Dixon G. H. The purification, characterization and partial sequence determination of a trout testis non-histone protein, HMG-T. Eur J Biochem. 1977 Mar 15;74(1):53–60. doi: 10.1111/j.1432-1033.1977.tb11365.x. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]