Abstract
We have determined the DNA sequences which correspond to the splicing regions in the transcripts of human papovavirus BKV, an evolutionary variant of SV40. To precisely localize the excision points in the BKV sequence, we have conducted a preliminary analysis of numerous viral and eukaryotic splice site sequences. This analysis suggests that the preferred sequence for the donor site belongs to at least one of four groups: Pu GTAxG, Pu GTAxxT, Pu GTxx GT, Pu GTxAG (= the cleavage site). These four groups derive from the two basic sequences, Pu GTxxG and Pu GTA. An optimal donor site might be: AG GTAAGT. The preferred sequence for the acceptor site is of the form PyPyxxPyAG; no dinucleotide AG occurs within 13 nucleotides prior to the terminal AG of the 3' end of the intervening sequences. As we have found in this study of BKV splicing RNA splice points when the DNA sequence is known.
Full text
PDF

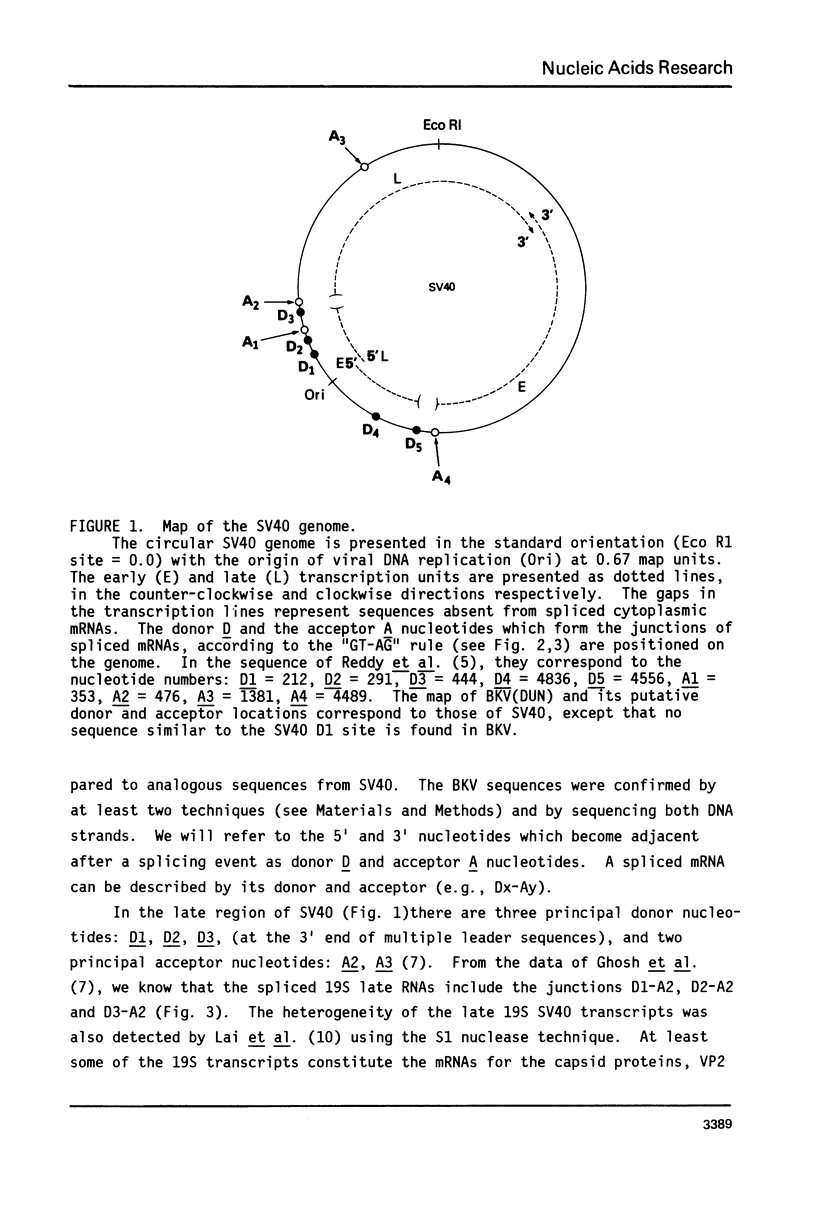
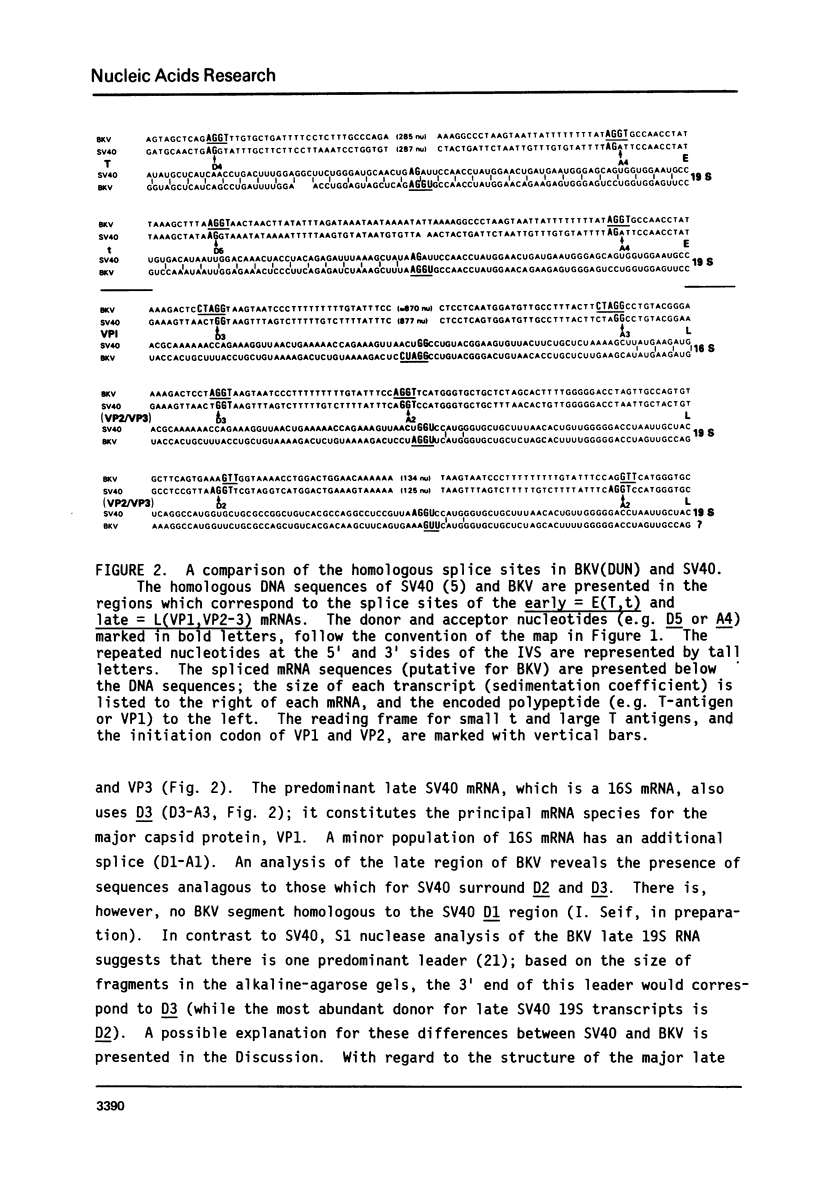
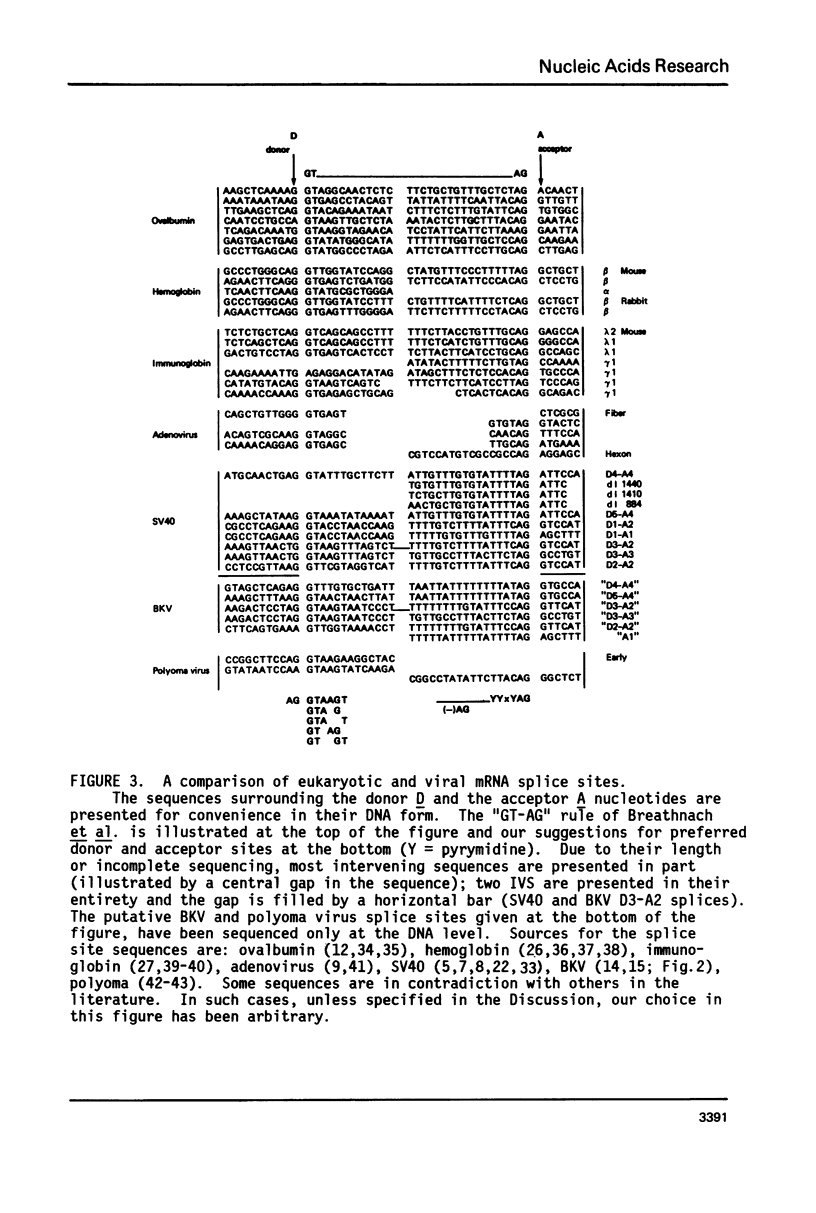







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Pettersson U. Nucleotide sequence at the junction between the coding region of the adenovirus 2 hexon messenger RNA and its leader sequence. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5822–5826. doi: 10.1073/pnas.75.12.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y., Shani M., Reuveni Y. RNAs of simian virus 40 in productively infected monkey cells: kinetics of formation and decay in enucleate cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2587–2591. doi: 10.1073/pnas.72.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Hozumi N., Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978 Dec;15(4):1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Bina-Stein M., Thoren M., Salzman N., Thomspon J. A. Rapid sequence determination of late simian virus 40 16S mRNA leader by using inhibitors of reverse transcriptase. Proc Natl Acad Sci U S A. 1979 Feb;76(2):731–735. doi: 10.1073/pnas.76.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall J. F., O'Malley B. W., Robertson M. A., Staden R., Tanaka Y., Brownlee G. G. Nucleotide sequence homology at 12 intron--exon junctions in the chick ovalbumin gene. Nature. 1978 Oct 12;275(5680):510–513. doi: 10.1038/275510a0. [DOI] [PubMed] [Google Scholar]
- Celma M. L., Dhar R., Pan J., Weissman S. M. Comparison of the nucleotide sequence of the messenger RNA for the major structural protein of SV40 with the DNA sequence encoding the amino acids of the protein. Nucleic Acids Res. 1977 Aug;4(8):2549–2550. [PMC free article] [PubMed] [Google Scholar]
- Crick F. Split genes and RNA splicing. Science. 1979 Apr 20;204(4390):264–271. doi: 10.1126/science.373120. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Implications of RNA-RNA splicing in evolution of eukaryotic cells. Science. 1978 Dec 22;202(4374):1257–1260. doi: 10.1126/science.364651. [DOI] [PubMed] [Google Scholar]
- Dhar R., Lai C. J., Khoury G. Nucleotide sequence of the DNA replication origin for human papovavirus BKV: sequence and structural homology with SV40. Cell. 1978 Feb;13(2):345–358. doi: 10.1016/0092-8674(78)90203-9. [DOI] [PubMed] [Google Scholar]
- Dhar R., Seif I., Khoury G. Nucleotide sequence of the BK virus DNA segment encoding small t antigen. Proc Natl Acad Sci U S A. 1979 Feb;76(2):565–569. doi: 10.1073/pnas.76.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Subramanian K. N., Pan J., Weissman S. M. Structure of a large segment of the genome of simian virus 40 that does not encode known proteins. Proc Natl Acad Sci U S A. 1977 Mar;74(3):827–831. doi: 10.1073/pnas.74.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Gannon F., O'Hare K., Perrin F., LePennec J. P., Benoist C., Cochet M., Breathnach R., Royal A., Garapin A., Cami B. Organisation and sequences at the 5' end of a cloned complete ovalbumin gene. Nature. 1979 Mar 29;278(5703):428–434. doi: 10.1038/278428a0. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Choudary P. V., Lebowitz P., Weissman S. M. The 5'-terminal leader sequence of late 16 S mRNA from cells infected with simian virus 40. J Biol Chem. 1978 May 25;253(10):3643–3647. [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Lebowitz P., Weissman S. M. Heterogeneity and 5'-terminal structures of the late RNAs of simian virus 40. J Mol Biol. 1978 Dec 25;126(4):813–846. doi: 10.1016/0022-2836(78)90022-0. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Hattori J., Carmichael G. G., Benjamin T. L. DNA sequence alterations in Hr-t deletion mutants of polyoma virus. Cell. 1979 Mar;16(3):505–513. doi: 10.1016/0092-8674(79)90025-4. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Konkel D. A., Tilghman S. M., Leder P. The sequence of the chromosomal mouse beta-globin major gene: homologies in capping, splicing and poly(A) sites. Cell. 1978 Dec;15(4):1125–1132. doi: 10.1016/0092-8674(78)90040-5. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Dhar R., Khoury G. Mapping the spliced and unspliced late lytic SV40 RNAs. Cell. 1978 Aug;14(4):971–982. doi: 10.1016/0092-8674(78)90351-3. [DOI] [PubMed] [Google Scholar]
- Leder A., Miller H. I., Hamer D. H., Seidman J. G., Norman B., Sullivan M., Leder P. Comparison of cloned mouse alpha- and beta-globin genes: conservation of intervening sequence locations and extragenic homology. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6187–6191. doi: 10.1073/pnas.75.12.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maat J., Smith A. J. A method for sequencing restriction fragments with dideoxynucleoside triphosphates. Nucleic Acids Res. 1978 Dec;5(12):4537–4545. doi: 10.1093/nar/5.12.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Weissman S. M. Gaps and duplicated sequences in the leaders of SV40 16S RNA. Nucleic Acids Res. 1978 Nov;5(11):4195–4213. doi: 10.1093/nar/5.11.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Sakano H., Rogers J. H., Hüppi K., Brack C., Traunecker A., Maki R., Wall R., Tonegawa S. Domains and the hinge region of an immunoglobulin heavy chain are encoded in separate DNA segments. Nature. 1979 Feb 22;277(5698):627–633. doi: 10.1038/277627a0. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Soeda E., Griffin B. E. Sequences from the genome of a non-transforming mutant of polyoma virus. Nature. 1978 Nov 16;276(5685):294–298. doi: 10.1038/276294a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Maxam A. M., Tizard R., Bernard O., Gilbert W. Sequence of a mouse germ-line gene for a variable region of an immunoglobulin light chain. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1485–1489. doi: 10.1073/pnas.75.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA sequence: extent of homology with simian virus 40 DNA. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1179–1183. doi: 10.1073/pnas.76.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg J., van Ooyen A., Mantei N., Schamböck A., Grosveld G., Flavell R. A., Weissmann C. Comparison of cloned rabbit and mouse beta-globin genes showing strong evolutionary divergence of two homologous pairs of introns. Nature. 1978 Nov 2;276(5683):37–44. doi: 10.1038/276037a0. [DOI] [PubMed] [Google Scholar]


