Abstract
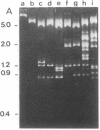
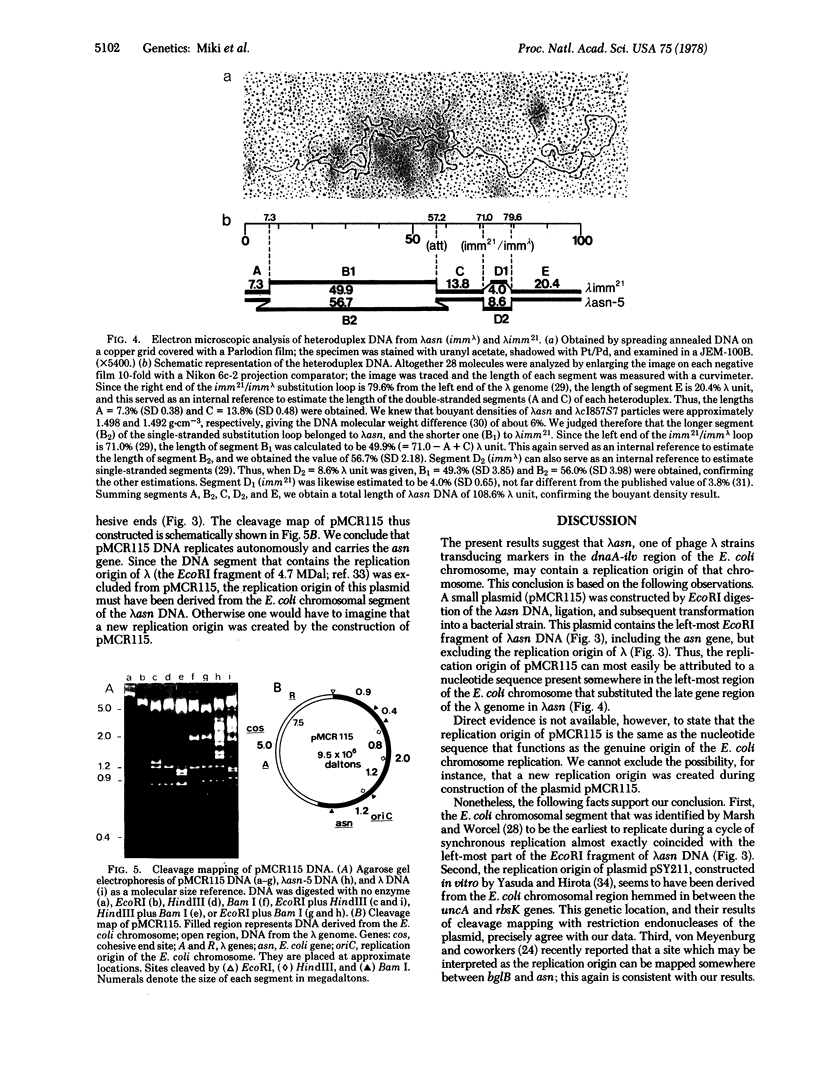
A transducing phage lambdaasn was isolated. The late gene region of its genome was found to have been substituted by an Escherichia coli chromosomal segment containing the genes bgIR, bgIC, glmS, uncA, and asn. Restriction endonuclease cleavage mapping and electron microscopic analysis of the lambdaasn DNA revealed that the size of the bacterial segment is approximately 1.75 X 10(7) daltons, corresponding to about 26.4 kilobases. The circular DNA of lambdaasn was digested with restriction endonuclease EcoRI, diluted, and sealed with DNA ligase. When the reaction mixture was used to transform a recipient E. coli strain, a small plasmid of about 1 X 10(7) daltons (named pMCR115) was obtained. Restriction endonuclease cleavage mapping of pMCR115 and other evidence suggested that it contained the replication origin (oriC) of the E. coli chromosome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird R. E., Louarn J., Martuscelli J., Caro L. Origin and sequence of chromosome replication in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):549–566. doi: 10.1016/0022-2836(72)90559-1. [DOI] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. The asparagine synthetase of Escherhic coli. I. Biosynthetic role of the enzyme, purification, and characterization of the reaction products. J Biol Chem. 1969 Aug 10;244(15):4112–4121. [PubMed] [Google Scholar]
- Chow L. T., Davidson N. Electron microscope study of the structures of lambdadv DNAs. J Mol Biol. 1974 Jun 15;86(1):69–89. doi: 10.1016/s0022-2836(74)80008-2. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Parkinson J. S. Deletion mutants of bacteriophage lambda. 3. Physical structure of att-phi. J Mol Biol. 1971 Mar 14;56(2):403–423. doi: 10.1016/0022-2836(71)90473-6. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Blattner F. R., McLeester C., Dove W. F. Genetic structure of the replication origin of bacteriophage lambda. Science. 1977 Dec 9;198(4321):1046–1051. doi: 10.1126/science.929186. [DOI] [PubMed] [Google Scholar]
- Haggerty D. M., Scheif R. F. Location in bacteriophage lamdba DNA of cleavage sites of the site-specific endonuclease from Bacillus amyloliquefaciens H. J Virol. 1976 May;18(2):659–663. doi: 10.1128/jvi.18.2.659-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S. Novel F prime factors able to replicate in Escherichia coli Hfr strains. Proc Natl Acad Sci U S A. 1976 Jan;73(1):198–202. doi: 10.1073/pnas.73.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld R., Vielmetter W. Bidirectional growth of the E. coli chromosome. Nat New Biol. 1973 Apr 4;242(118):130–132. doi: 10.1038/newbio242130a0. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. I. Molecular origin of the fragments. J Mol Biol. 1965 Nov;14(1):85–109. doi: 10.1016/s0022-2836(65)80232-7. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T., Yura T., Yamagishi H. Genetic and physical studies of lambda transducing bacteriophage carrying the beta subunit gene of the Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1975 Jun;122(3):1247–1256. doi: 10.1128/jb.122.3.1247-1256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Landy A., Ross W. Viral integration and excision: structure of the lambda att sites. Science. 1977 Sep 16;197(4309):1147–1160. doi: 10.1126/science.331474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louarn J., Funderburgh M., Bird R. E. More precise mapping of the replication origin in Escherichia coli K-12. J Bacteriol. 1974 Oct;120(1):1–5. doi: 10.1128/jb.120.1.1-5.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R. C., Worcel A. A DNA fragment containing the origin of replication of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2720–2724. doi: 10.1073/pnas.74.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Meselson M. Periodic replication of DNA in steadily growing Escherichia coli: the localized origin of replication. Cold Spring Harb Symp Quant Biol. 1968;33:553–557. doi: 10.1101/sqb.1968.033.01.062. [DOI] [PubMed] [Google Scholar]
- Prasad I., Schaefler S. Regulation of the beta-glucoside system in Escherchia coli K-12. J Bacteriol. 1974 Nov;120(2):638–650. doi: 10.1128/jb.120.2.638-650.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L. H., Landy A. HindII, HindIII, and HpaI restriction fragment maps of bacteriophage lambda DNA. Gene. 1977 Sep;2(1):1–31. doi: 10.1016/0378-1119(77)90019-1. [DOI] [PubMed] [Google Scholar]
- Saito T., Hiraga S. Initiation of DNA replication in Escherichia coli. III. Genetic analysis of the dna mutant exhibiting rifampicin-sensitive resumption of replication. Mol Gen Genet. 1975;137(3):249–261. doi: 10.1007/BF00333020. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. III. The components of the secondary attachment sites. J Mol Biol. 1975 Apr 25;93(4):415–429. doi: 10.1016/0022-2836(75)90237-5. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Hirota Y. Cloning and mapping of the replication origin of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5458–5462. doi: 10.1073/pnas.74.12.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Hansen F. G., Nielsen L. D., Jørgensen P. Origin of replication, oriC, of the Escherichia coli chromosome: mapping of genes relative to R.EcoRI cleavage sites in the oriC region. Mol Gen Genet. 1977 Dec 14;158(1):101–109. doi: 10.1007/BF00455124. [DOI] [PubMed] [Google Scholar]