Abstract
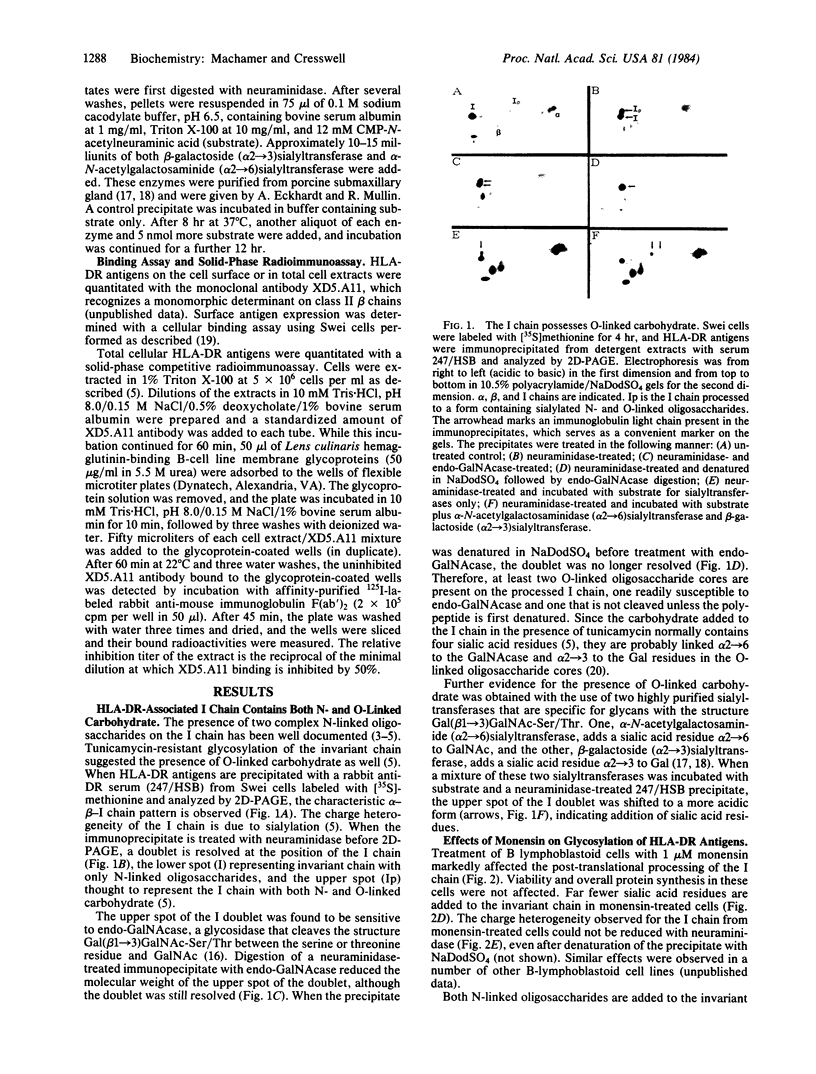
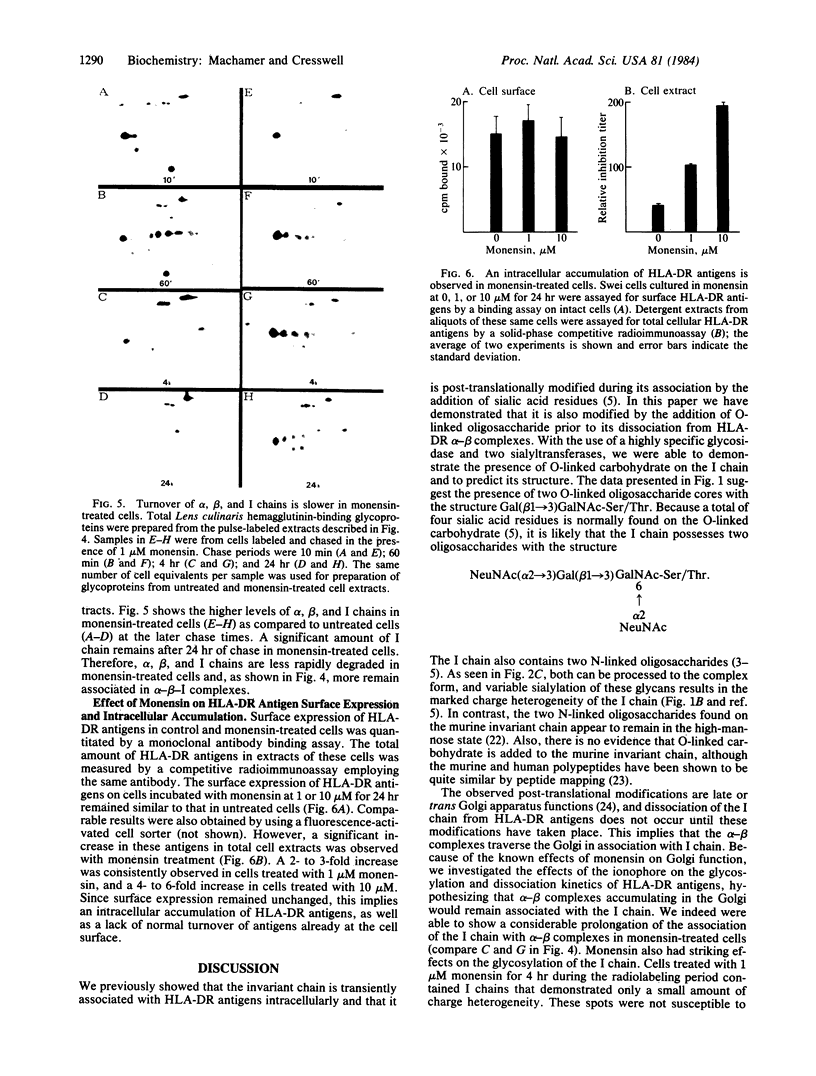
In B-lymphoblastoid cells, the HLA-DR-associated invariant chain is processed to a form containing O-linked as well as N-linked oligosaccharides. After neuraminidase treatment, the O-linked carbohydrate is susceptible to digestion with an endoglycosidase (endo-beta-N-acetylgalactosaminidase) that cleaves glycans with the structure Gal(beta 1----3)-GalNAc-Ser/Thr, and sialic acid can be added back to this core oligosaccharide by specific sialyltransferases. Treatment of cells with the sodium ionophore monensin markedly affects the post-translational processing of the invariant chain, although that of associated alpha and beta chains is minimally affected. Only a small portion of the N-linked carbohydrate on the invariant chain is processed to an endoglycosidase-H-resistant form. The sialic acid residues normally found on the O-linked glycans are not added, but at least the first residue, GalNAc, is added. In addition to the changes in glycosylation, an intracellular accumulation of HLA-DR antigens also occurs in monensin-treated cells. The accumulation of HLA-DR antigens and the overall slower turnover rates of the alpha, beta, and invariant polypeptides observed after monensin treatment probably reflects the build-up of newly synthesized proteins in Golgi apparatus-derived vacuoles coupled with a decrease in normal degradation in lysosomes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer T. A., Rearick J. I., Paulson J. C., Prieels J. P., Sadler J. E., Hill R. L. Biosynthesis of mammalian glycoproteins. Glycosylation pathways in the synthesis of the nonreducing terminal sequences. J Biol Chem. 1979 Dec 25;254(24):12531–12534. [PubMed] [Google Scholar]
- Charron D. J., Aellen-Schulz M. F., St Geme J., 3rd, Erlich H. A., McDevitt H. O. Biochemical characterization of an invariant polypeptide associated with Ia antigens in human and mouse. Mol Immunol. 1983 Jan;20(1):21–32. doi: 10.1016/0161-5890(83)90101-3. [DOI] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Analysis of HLA-D region-associated molecules with monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6567–6571. doi: 10.1073/pnas.76.12.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow L. R., Paulson J. C., Hill R. L. Systematic purification of five glycosidases from Streptococcus (Diplococcus) pneumoniae. J Biol Chem. 1977 Dec 10;252(23):8615–8623. [PubMed] [Google Scholar]
- Griffiths G., Quinn P., Warren G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J Cell Biol. 1983 Mar;96(3):835–850. doi: 10.1083/jcb.96.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford J., Wolkoff A. W., Ashwell G., Klausner R. D. Monensin inhibits intracellular dissociation of asialoglycoproteins from their receptor. J Cell Biol. 1983 Jun;96(6):1824–1828. doi: 10.1083/jcb.96.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., Hewgill D., McDevitt H. O. Detection of a common polypeptide chain in I--A and I--E sub-region immunoprecipitates. Mol Immunol. 1979 Jan;16(1):51–60. doi: 10.1016/0161-5890(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Koch N., Hämmerling G. J., Szymura J., Wabl M. R. Ia associated Ii chain is not encoded by chromosome 17 of the mouse. Immunogenetics. 1982;16(6):603–606. doi: 10.1007/BF00372029. [DOI] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Long E. O., Strubin M., Wake C. T., Gross N., Carrel S., Goodfellow P., Accolla R. S., Mach B. Isolation of cDNA clones for the p33 invariant chain associated with HLA-DR antigens. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5714–5718. doi: 10.1073/pnas.80.18.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Cresswell P. Biosynthesis and glycosylation of the invariant chain associated with HLA-DR antigens. J Immunol. 1982 Dec;129(6):2564–2569. [PubMed] [Google Scholar]
- Moosic J. P., Nilson A., Hämmerling G. J., McKean D. J. Biochemical characterization of Ia antigens. I. Characterization of the 31K polypeptide associated with I-A subregion Ia antigens. J Immunol. 1980 Oct;125(4):1463–1469. [PubMed] [Google Scholar]
- Murphy L. A., Goldstein I. J. Five alpha-D-galactopyranosyl-binding isolectins from Bandeiraea simplicifolia seeds. J Biol Chem. 1977 Jul 10;252(13):4739–4742. [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F., Crumpton M. J. Biosynthesis and maturation of HLA-DR antigens in vivo. J Biol Chem. 1981 Sep 10;256(17):8987–8993. [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Quinn P., Griffiths G., Warren G. Dissection of the Golgi complex. II. Density separation of specific Golgi functions in virally infected cells treated with monensin. J Cell Biol. 1983 Mar;96(3):851–856. doi: 10.1083/jcb.96.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radka S. F., Machamer C., Cresswell P., Kostyu D. D., Ward F. E., Amos D. B. SFR3-DR5, a monoclonal antibody with HLA-DR5 specificity. J Immunol. 1983 Apr;130(4):1863–1866. [PubMed] [Google Scholar]
- Rothman J. E. The golgi apparatus: two organelles in tandem. Science. 1981 Sep 11;213(4513):1212–1219. doi: 10.1126/science.7268428. [DOI] [PubMed] [Google Scholar]
- Sadler J. E., Rearick J. I., Hill R. L. Purification to homogeneity and enzymatic characterization of an alpha-N-acetylgalactosaminide alpha 2 leads to 6 sialyltransferase from porcine submaxillary glands. J Biol Chem. 1979 Jul 10;254(13):5934–5941. [PubMed] [Google Scholar]
- Sadler J. E., Rearick J. I., Paulson J. C., Hill R. L. Purification to homogeneity of a beta-galactoside alpha2 leads to 3 sialyltransferase and partial purification of an alpha-N-acetylgalactosaminide alpha2 leads to 6 sialyltransferase from porcine submaxillary glands. J Biol Chem. 1979 Jun 10;254(11):4434–4442. [PubMed] [Google Scholar]
- Shackelford D. A., Kaufman J. F., Korman A. J., Strominger J. L. HLA-DR antigens: structure, separation of subpopulations, gene cloning and function. Immunol Rev. 1982;66:133–187. doi: 10.1111/j.1600-065x.1982.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Sung E., Jones P. P. The invariant chain of murine Ia antigens: its glycosylation, abundance and subcellular localization. Mol Immunol. 1981 Oct;18(10):899–913. doi: 10.1016/0161-5890(81)90013-4. [DOI] [PubMed] [Google Scholar]
- Swiedler S. J., Hart G. W., Freed J. H. Characterization of the oligosaccharides from the invariant chain associated with murine Ia antigens. J Immunol. 1983 Jul;131(1):352–358. [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Tartakoff A., Hoessli D., Vassalli P. Intracellular transport of lymphoid surface glycoproteins. Role of the Golgi complex. J Mol Biol. 1981 Aug 25;150(4):525–535. doi: 10.1016/0022-2836(81)90378-8. [DOI] [PubMed] [Google Scholar]
- Yamashiro D. J., Fluss S. R., Maxfield F. R. Acidification of endocytic vesicles by an ATP-dependent proton pump. J Cell Biol. 1983 Sep;97(3):929–934. doi: 10.1083/jcb.97.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]