Abstract
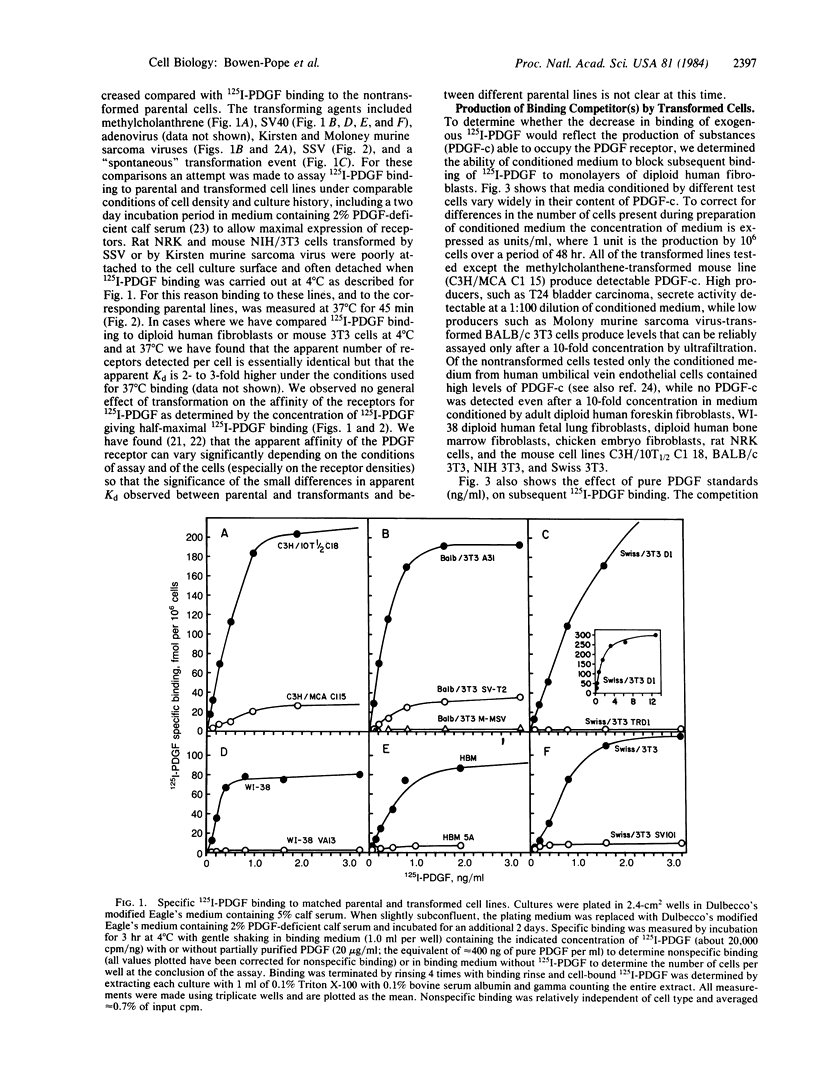
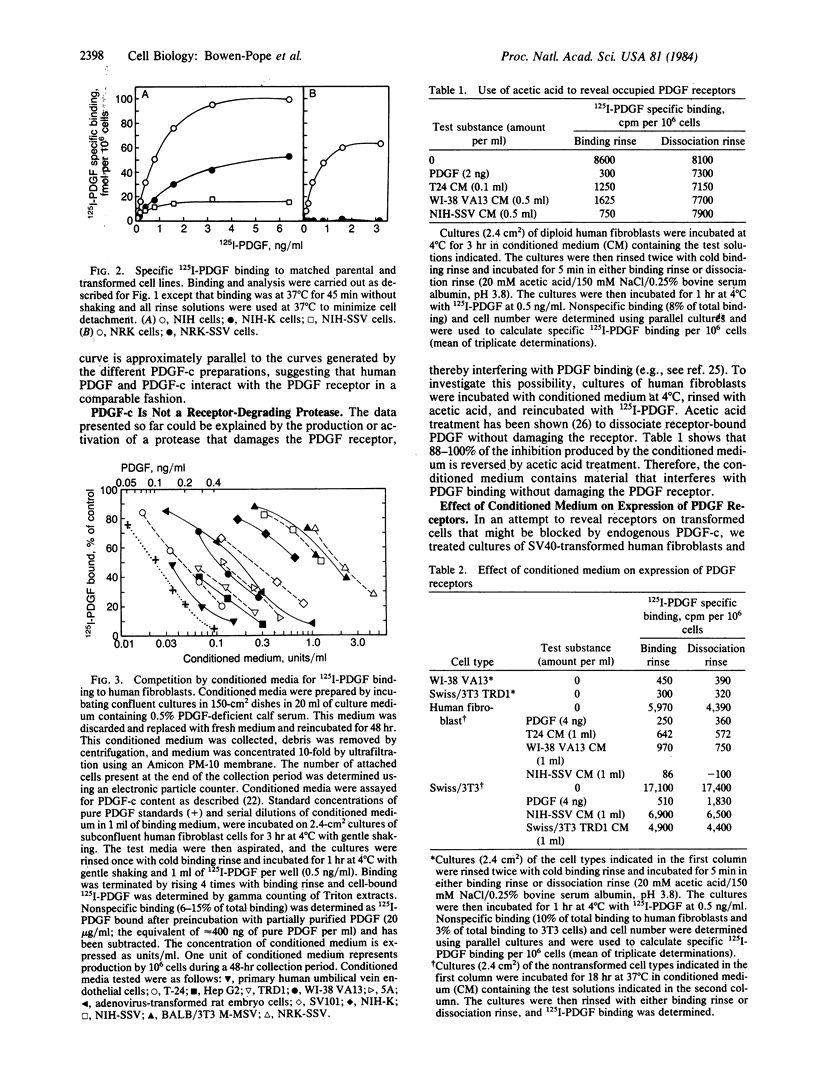
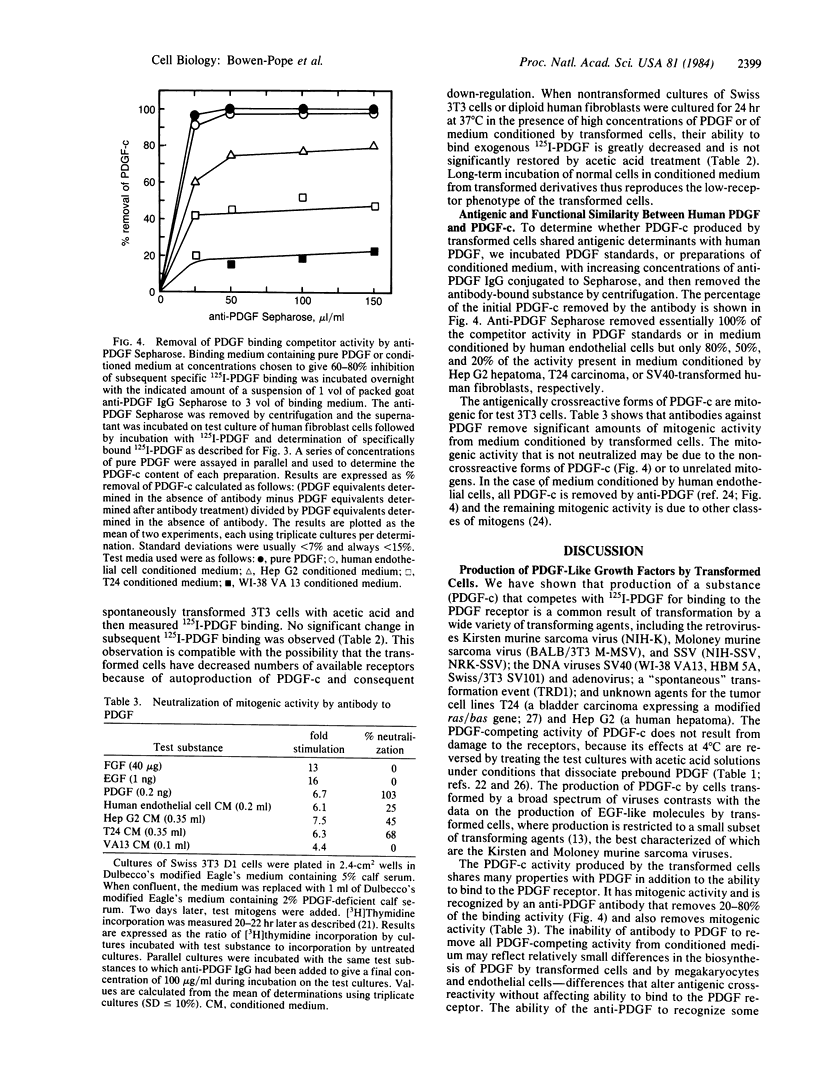
A series of nontransformed human and murine cells and derivative cell lines transformed by methylcholanthrene; by simian virus 40, Kirsten and Moloney murine sarcoma viruses, simian sarcoma virus, and adenovirus; and by a "spontaneous" event in culture were examined for the expression of receptors for the platelet-derived growth factor (PDGF) and for production of substances able to compete with 125I-labeled PDGF for binding to the cell-surface PDGF receptor. In each case, transformation resulted in a 50-100% decrease in available PDGF receptors. All transformed cells except the methylcholanthrene-transformed mouse cells produce a PDGF competitor into the conditioned medium. Levels of PDGF competitor in conditioned medium at the end of a 48-hr collection were as high as 2 ng/ml--high enough to be measured by radioreceptor assay diluted 1:30 and to maximally stimulate [3H]thymidine incorporation by human fibroblasts. The PDGF competitor activity detected in a radioreceptor assay does not reflect irreversible (e.g., proteolytic) damage to the receptor of test cells since its effects are reversed by acetic acid dissociation. Antiserum against human PDGF neutralizes 20-80% of the PDGF competitor found in conditioned medium from different transformed human cells and 100% of the activity from normal human endothelial cells. The possibility that induction of expression of the cellular PDGF gene may be involved in the mechanism of transformation of PDGF-responsive mesenchymal cells is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. R., Devare S. G., Tronick S. R., Ellis R. W., Aaronson S. A., Scolnick E. M. Generation of BALB-MuSV and Ha-MuSC by type C virus transduction of homologous transforming genes from different species. Cell. 1981 Oct;26(1 Pt 1):129–134. doi: 10.1016/0092-8674(81)90041-6. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Dicorleto P. E., Ross R. Interactions between the receptors for platelet-derived growth factor and epidermal growth factor. J Cell Biol. 1983 Mar;96(3):679–683. doi: 10.1083/jcb.96.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Platelet-derived growth factor. II. Specific binding to cultured cells. J Biol Chem. 1982 May 10;257(9):5161–5171. [PubMed] [Google Scholar]
- Clarke G. D., Stoker M. G., Ludlow A., Thornton M. Requirement of serum for DNA synthesis in BHK 21 cells: effects of density, suspension and virus transformation. Nature. 1970 Aug 22;227(5260):798–801. doi: 10.1038/227798a0. [DOI] [PubMed] [Google Scholar]
- Currie G. A. Platelet-derived growth-factor requirements for in vitro proliferation of normal and malignant mesenchymal cells. Br J Cancer. 1981 Mar;43(3):335–343. doi: 10.1038/bjc.1981.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Huang S. S., Stroobant P., Waterfield M. D. Expression of a platelet-derived growth factor-like protein in simian sarcoma virus transformed cells. Science. 1983 Sep 30;221(4618):1348–1350. doi: 10.1126/science.6310754. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker P., Pohjanpelto P., Pettican P., Rozengurt E. Similarities between fibroblast-derived growth factor and platelet-derived growth factor. Exp Cell Res. 1981 Sep;135(1):221–227. doi: 10.1016/0014-4827(81)90314-1. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Retroviral transforming genes in normal cells? Nature. 1983 Jul 21;304(5923):219–226. doi: 10.1038/304219a0. [DOI] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Owen A. J., Antoniades H. N. Evidence that a human osteosarcoma cell line which secretes a mitogen similar to platelet-derived growth factor requires growth factors present in platelet-poor plasma. Cancer Res. 1983 Jan;43(1):83–87. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Chemical and biological properties of a growth factor from human-cultured osteosarcoma cells: resemblance with platelet-derived growth factor. J Cell Physiol. 1980 Nov;105(2):235–246. doi: 10.1002/jcp.1041050207. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. L., Anderson M., Ozanne B. Transforming growth factor(s) production enables cells to grow in the absence of serum: an autocrine system. Proc Natl Acad Sci U S A. 1982 Jan;79(2):485–489. doi: 10.1073/pnas.79.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Nistér M., Heldin C. H., Wasteson A., Westermark B. A platelet-derived growth factor analog produced by a human clonal glioma cell line. Ann N Y Acad Sci. 1982 Dec 10;397:25–33. doi: 10.1111/j.1749-6632.1982.tb43414.x. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Scher C. D., Pledger W. J., Martin P., Antoniades H., Stiles C. D. Transforming viruses directly reduce the cellular growth requirement for a platelet derived growth factor. J Cell Physiol. 1978 Dec;97(3 Pt 1):371–380. doi: 10.1002/jcp.1040970312. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Cohen S. Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature. 1976 Nov 4;264(5581):26–31. doi: 10.1038/264026a0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A., Raines E., Kariya B., Rivest M. J., Ross R. Coordinate control of 3T3 cell proliferation by platelet-derived growth factor and plasma components. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2810–2814. doi: 10.1073/pnas.75.6.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]


