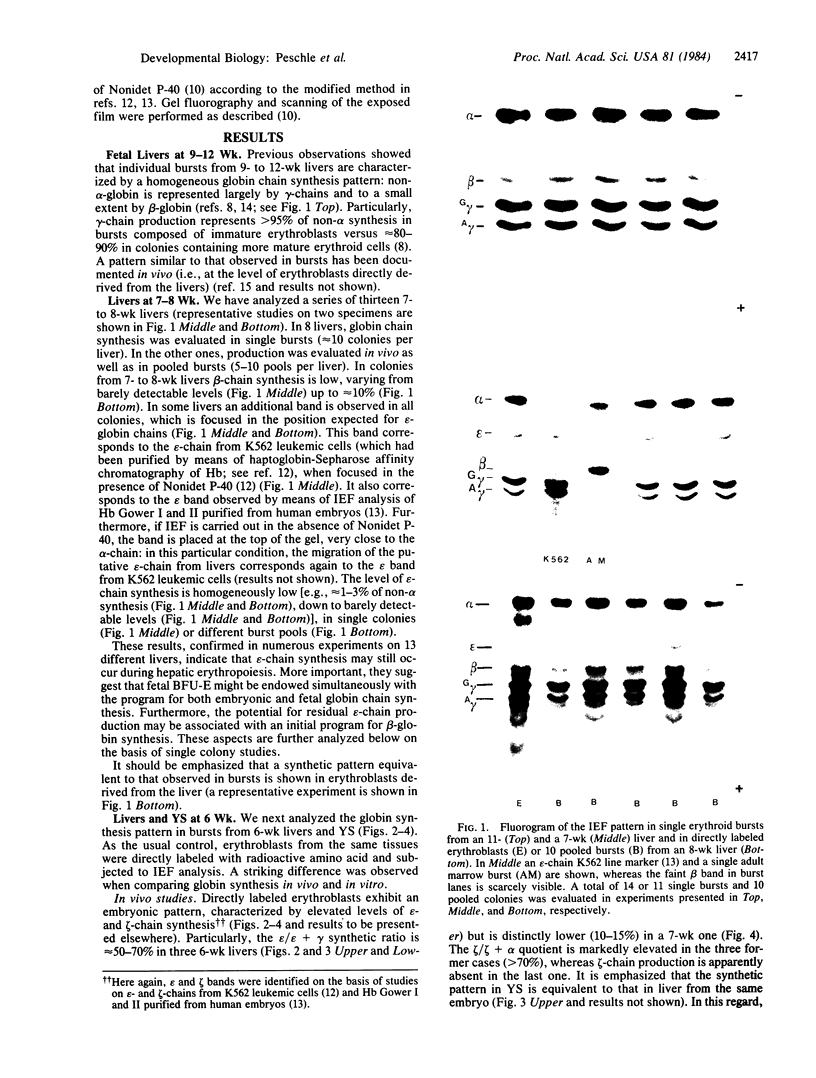
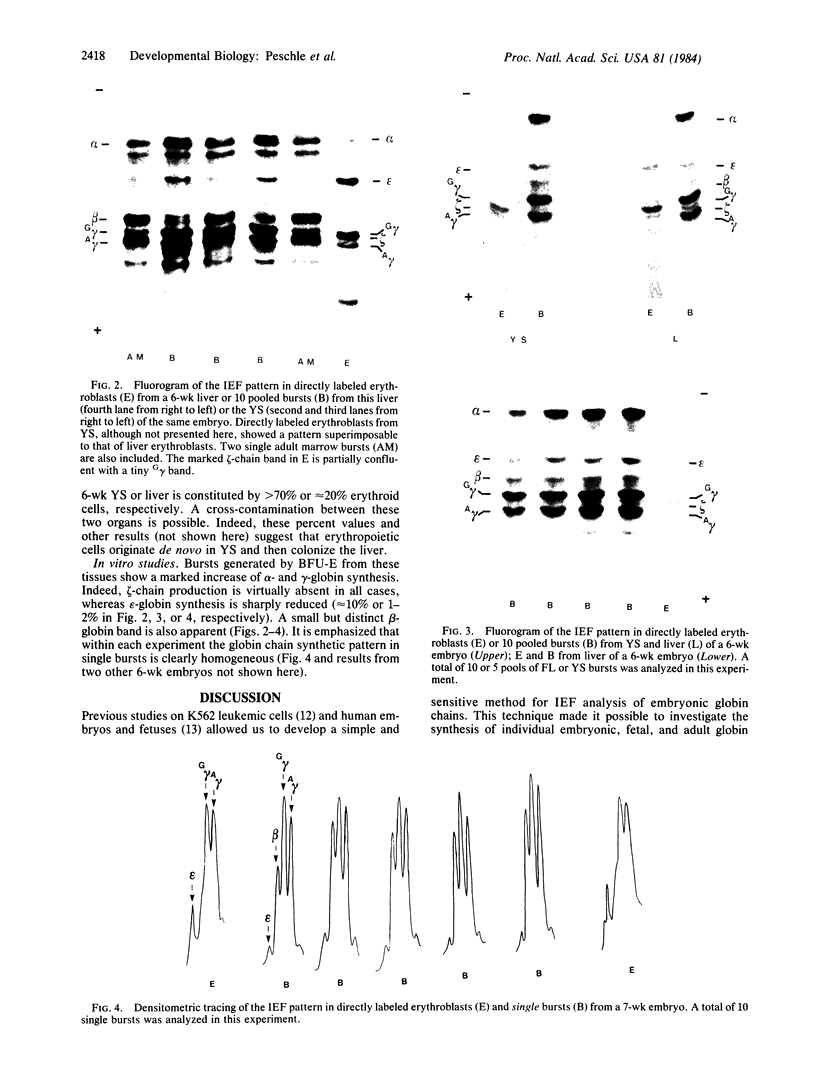
Abstract
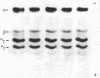
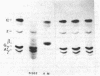
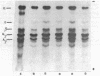
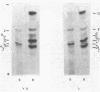
The synthesis of embryonic (zeta, epsilon), fetal (alpha, gamma), and adult (beta) globin was evaluated in human yolk sacs (YS) and livers at different ontogenic stages (i.e., from 6 through 10-12 wk of age) by means of analytical isoelectric focusing. Globin production was comparatively evaluated in vivo (i.e., in directly labeled erythroblasts from YS and liver) and in vitro [i.e., in erythroid bursts generated in culture by erythroid burst-forming units (BFU-E) from the same erythropoietic tissues]. Erythroid bursts generated in vitro by BFU-E from 6-wk livers and YS show essentially a "fetal" globin synthetic pattern: this is in sharp contrast to the "embryonic" pattern in corresponding liver and YS erythroblasts directly labeled in vivo. The invitro phenomenon suggests that (i) 6-wk BFU-E constitute a new generation of progenitors, which have already switched from an embryonic to a fetal program, and/or (ii) expression of their fetal program is induced by unknown in vitro factor(s), which may underlie the in vivo switch at later ontogenic stages. It is emphasized that 6- to 7-wk BFU-E are endowed with the potential for in vitro synthesis of not only epsilon- and gamma-chains but also some beta-globin. In general, we observed an inverse correlation between the levels of epsilon- and beta-chain synthesis. These results, together with previous studies on fetal, perinatal, and adult BFU-E, are compatible with models suggesting that in ontogeny the chromatin configuration is gradually modified at the level of the non-alpha gene cluster, thus leading to a 5'----3' activation of globin genes in a balanced fashion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Comi P., Giglioni B., Ottolenghi S., Gianni A. M., Polli E., Barba P., Covelli A., Migliaccio G., Condorelli M., Peschle C. Globin chain synthesis in single erythroid bursts from cord blood: studies on gamma leads to beta and G gamma leads to A gamma switches. Proc Natl Acad Sci U S A. 1980 Jan;77(1):362–365. doi: 10.1073/pnas.77.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudennec C. A., Thiery J. P., Le Douarin N. M. In vitro induction of adult erythropoiesis in early mouse yolk sac. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2412–2416. doi: 10.1073/pnas.78.4.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover G. J., Ogawa M. Cellular mechanisms for increased fetal hemoglobin production in culture. Evidence for continuous commitment to fetal hemoglobin production during burst formation. J Clin Invest. 1980 Nov;66(5):1175–1178. doi: 10.1172/JCI109949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale R. E., Clegg J. B., Huehns E. R. Human embryonic haemoglobins Gower 1 and Gower 2. Nature. 1979 Jul 12;280(5718):162–164. doi: 10.1038/280162a0. [DOI] [PubMed] [Google Scholar]
- Gianni A. M., Comi P., Giglioni B., Ottolenghi S., Migliaccio A. R., Migliaccio G., Lettieri F., Maguire Y. P., Peschle C. Biosynthesis of Hb in individual fetal liver bursts. gamma-Chain production peaks earlier than beta-chain in the erythropoietic pathway. Exp Cell Res. 1980 Dec;130(2):345–352. doi: 10.1016/0014-4827(80)90011-7. [DOI] [PubMed] [Google Scholar]
- Gianni A. M., Presta M., Polli E., Peschle C., Lettieri F., Saglio G., Comi P., Giglioni B., Ottolenghi S. Preferential induction of fetal versus embryonic globin chains in human leukemic cell lines. Leuk Res. 1982;6(2):155–163. doi: 10.1016/0145-2126(82)90021-2. [DOI] [PubMed] [Google Scholar]
- HUEHNS E. R., FLYNN F. V., BUTLER E. A., BEAVEN G. H. Two new haemoglobin variants in a very young human embryo. Nature. 1961 Feb 11;189:496–497. doi: 10.1038/189496a0. [DOI] [PubMed] [Google Scholar]
- Hecht F., Motulsky A. G., Lemire R. J., Shepard T. E. Predominance of hemoglobin Gower 1 in early human embryonic development. Science. 1966 Apr 1;152(3718):91–92. doi: 10.1126/science.152.3718.91. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Kaback M. M., Kazazian H. H., Jr Adult hemoglobin synthesis by reticulocytes from the human fetus at midtrimester. Science. 1971 Nov 12;174(4010):698–702. doi: 10.1126/science.174.4010.698. [DOI] [PubMed] [Google Scholar]
- Huehns E. R. The properties and reactions of haemoglobin F(1) and their bearing on the dissociation equilibrium of haemoglobin. Biochem J. 1966 Dec;101(3):852–860. doi: 10.1042/bj1010852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavilio F., Giampaolo A., Carè A., Migliaccio G., Calandrini M., Russo G., Pagliardi G. L., Mastroberardino G., Marinucci M., Peschle C. Molecular mechanisms of human hemoglobin switching: selective undermethylation and expression of globin genes in embryonic, fetal, and adult erythroblasts. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6907–6911. doi: 10.1073/pnas.80.22.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Kurachi S., Nakamoto B., Zanjani E. D., Stamatoyannopoulos G. Hemoglobin switching in culture: evidence for a humoral factor that induces switching in adult and neonatal but not fetal erythroid cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6579–6583. doi: 10.1073/pnas.79.21.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschle C., Migliaccio A. R., Migliaccio G., Ciccariello R., Lettieri F., Quattrin S., Russo G., Mastroberardino G. Identification and characterization of three classes of erythroid progenitors in human fetal liver. Blood. 1981 Sep;58(3):565–572. [PubMed] [Google Scholar]
- Peschle C., Migliaccio G., Covelli A., Lettieri F., Migliaccio A. R., Condorelli M., Comi P., Pozzoli M. L., Giglioni B., Ottolenghi S. Hemoglobin synthesis in individual bursts from normal adult blood: all bursts and subcolonies synthesize G gamma-and A gamma-globin chains. Blood. 1980 Aug;56(2):218–226. [PubMed] [Google Scholar]
- Presta M., Giglioni B., Ottolenghi S., Gianni A. M., Capaldi A., Trento M., Saglio G. Analysis of human embryonic hemoglobins and globins by isoelectric focusing. Haematologica. 1983 Jul-Aug;68(4):443–453. [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Rosenblum B. B., Papayannopoulou T., Brice M., Nakamoto B., Shepard T. H. HbF and HbA production in erythroid cultures from human fetuses and neonates. Blood. 1979 Aug;54(2):440–450. [PubMed] [Google Scholar]
- Terasawa T., Ogawa M., Porter P. N., Karam J. D. G gamma and A gamma globin-chain biosynthesis by adult and umbilical cord blood erythropoietic bursts and reticulocytes. Blood. 1980 Jul;56(1):93–97. [PubMed] [Google Scholar]
- Wong P. M., Clarke B. J., Carr D. H., Chui D. H. Adult hemoglobins are synthesized in erythroid colonies in vitro derived from murine circulating hemopoietic progenitor cells during embryonic development. Proc Natl Acad Sci U S A. 1982 May;79(9):2952–2956. doi: 10.1073/pnas.79.9.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]