Abstract
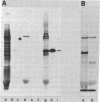
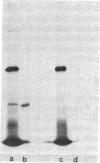
A monoclonal antibody (HC 2.1) directed against the separated heavy chain of HLA-DR has been prepared. By binding HC 2.1 to polysomes from human B lymphoblastoid cells followed by the use of a protein A-Sepharose column as an immunoadsorbent, we have purified the mRNA coding for the HLA-DR heavy chain nearly to homogeneity. The immunopurified mRNA has been used to prepare labeled cDNA with which to probe cDNA libraries. Double-stranded cDNA was also made from the immunopurified mRNA and cloned directly into pBR322. Two clones, one from each of the above procedures, positively selected DR heavy chain message as assayed by cell-free translation and immunoprecipitation. One clone, pDRH-2 [500 base pairs plus 75 base pairs of poly(A)] contains the entire 3' untranslated region as well as coding information for the carboxy-terminal hydrophilic intracellular domain and part of the hydrophobic transmembrane region. Results of carboxypeptidase digestion of the heavy chains from detergent-solubilized (p34) and papain-treated (p33) HLA-DR antigen were consistent with the predicted protein sequence. Specific immunopurification of polysomes by defined monoclonal antibodies followed by direct cloning of cDNA to the highly purified mRNA is a powerful method for obtaining identified cDNA clones.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- COWIE D. B., SPIEGELMAN S., ROBERTS R. B., DUERKSEN J. D. Ribosome-bound beta-galactosidase. Proc Natl Acad Sci U S A. 1961 Jan 15;47:114–122. doi: 10.1073/pnas.47.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cami B., Brégégère F., Abastado J. P., Kourilsky P. Multiple sequences related to classical histocompatibility antigens in the mouse genome. Nature. 1981 Jun 25;291(5817):673–675. doi: 10.1038/291673a0. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Uehara H., Martinko J., Nathenson S. G. Primary structure of a murine transplantation antigen. Nature. 1981 May 7;291(5810):35–39. doi: 10.1038/291035a0. [DOI] [PubMed] [Google Scholar]
- Corte G., Calabi F., Damiani G., Bargellesi A., Tosi R., Sorrentino R. Human Ia molecules carrying DC1 determinants differ in both alpha- and beta-subunits from Ia molecules carrying DR determinants. Nature. 1981 Jul 23;292(5821):357–360. doi: 10.1038/292357a0. [DOI] [PubMed] [Google Scholar]
- Cundliffe E., Cannon M., Davies J. Mechanism of inhibition of eukaryotic protein synthesis by trichothecene fungal toxins. Proc Natl Acad Sci U S A. 1974 Jan;71(1):30–34. doi: 10.1073/pnas.71.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Derynck R., Remaut E., Saman E., Stanssens P., De Clercq E., Content J., Fiers W. Expression of human fibroblast interferon gene in Escherichia coli. Nature. 1980 Sep 18;287(5779):193–197. doi: 10.1038/287193a0. [DOI] [PubMed] [Google Scholar]
- Flick P. K., Chen J., Alberts A. W., Vagelos P. R. Translation of rat liver fatty acid synthetase mRNA in a cell-free system derived from wheat germ. Proc Natl Acad Sci U S A. 1978 Feb;75(2):730–734. doi: 10.1073/pnas.75.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudswaard J., van der Donk J. A., Noordzij A., van Dam R. H., Vaerman J. P. Protein A reactivity of various mammalian immunoglobulins. Scand J Immunol. 1978;8(1):21–28. doi: 10.1111/j.1365-3083.1978.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Adams J. M. Immunoprecipitation of specific polysomes using Staphylococcus aureus: purification of the immunoglobulin k chain messenger RNA from the mouse myeloma MPC11. Biochemistry. 1978 Dec 12;17(25):5560–5566. doi: 10.1021/bi00618a036. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. Studies on the selectivity of DNA precipitation by spermine. Nucleic Acids Res. 1981 Oct 24;9(20):5493–5504. doi: 10.1093/nar/9.20.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J. F., Strominger J. L. Both chains of HLA-DR bind to the membrane with a penultimate hydrophobic region and the heavy chain is phosphorylated at its hydrophilic carboxyl terminus. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6304–6308. doi: 10.1073/pnas.76.12.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Ploegh H. L., Kaufman J. F., Owen M. J., Strominger J. L. Cell-free synthesis and processing of the heavy and light chains of HLA-DR antigens. J Exp Med. 1980 Aug 1;152(2 Pt 2):65s–82s. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Palacios R., Palmiter R. D., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. I. Specific binding of 125 I-anti-ovalbumin to polysomes. J Biol Chem. 1972 Apr 25;247(8):2316–2321. [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Cannon L. E., Strominger J. L. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2273–2277. doi: 10.1073/pnas.76.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Molecular cloning of a human histocompatibility antigen cDNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6081–6085. doi: 10.1073/pnas.77.10.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I. Biologically and chemically pure mRNA coding for a mouse immunoglobulin L-chain prepared with the aid of antibodies and immobilized oligothymidine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2256–2260. doi: 10.1073/pnas.70.8.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz G., Kieval S., Groner B., Sippel A. E., Kurtz D., Feigelson P. Isolation of specific messenger RNA by adsorption of polysomes to matrix-bound antibody. Nucleic Acids Res. 1977 Jan;4(1):71–84. doi: 10.1093/nar/4.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. C., Wells J. R. The isolation of chicken histone F2c(v) messenger RNA by immunoadsorption of F2c-synthesising polysomes. Biochem Biophys Res Commun. 1975 May 5;64(1):448–455. doi: 10.1016/0006-291x(75)90273-9. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Lampson L. A., Strominger J. L. Analysis of HLA-DR antigens by using monoclonal antibodies: recognition of conformational differences in biosynthetic intermediates. J Immunol. 1981 Oct;127(4):1403–1410. [PubMed] [Google Scholar]
- Shackelford D. A., Mann D. L., van Rood J. J., Ferrara G. B., Strominger J. L. Human B-cell alloantigens DC1, MT1, and LB12 are identical to each other but distinct from the HLA-DR antigen. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4566–4570. doi: 10.1073/pnas.78.7.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. Z., Young J. R. An immunochemical method for mRNA purification. Application to messenger RNA encoding trypanosome variable surface antigen. J Biol Chem. 1981 Feb 25;256(4):1495–1498. [PubMed] [Google Scholar]
- Steinmetz M., Frelinger J. G., Fisher D., Hunkapiller T., Pereira D., Weissman S. M., Uehara H., Nathenson S., Hood L. Three cDNA clones encoding mouse transplantation antigens: homology to immunoglobulin genes. Cell. 1981 Apr;24(1):125–134. doi: 10.1016/0092-8674(81)90508-0. [DOI] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- Uhr J. W., Capra J. D., Vitetta E. S., Cook R. G. Organization of the immune response genes. Science. 1979 Oct 19;206(4416):292–297. doi: 10.1126/science.113876. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Primary structure of the COOH-terminal membranous segment of a penicillin-sensitive enzyme purified from two Bacilli. J Biol Chem. 1981 Feb 25;256(4):2067–2077. [PubMed] [Google Scholar]
- Wei C. M., Hansen B. S., Vaughan M. H., Jr, McLaughlin C. S. Mechanism of action of the mycotoxin trichodermin, a 12,13-epoxytrichothecene. Proc Natl Acad Sci U S A. 1974 Mar;71(3):713–717. doi: 10.1073/pnas.71.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Woo S. L., Beattie W. G., Catterall J. F., Dugaiczyk A., Staden R., Brownlee G. G., O'Malley B. W. Complete nucleotide sequence of the chicken chromosomal ovalbumin gene and its biological significance. Biochemistry. 1981 Oct 27;20(22):6437–6446. doi: 10.1021/bi00525a024. [DOI] [PubMed] [Google Scholar]