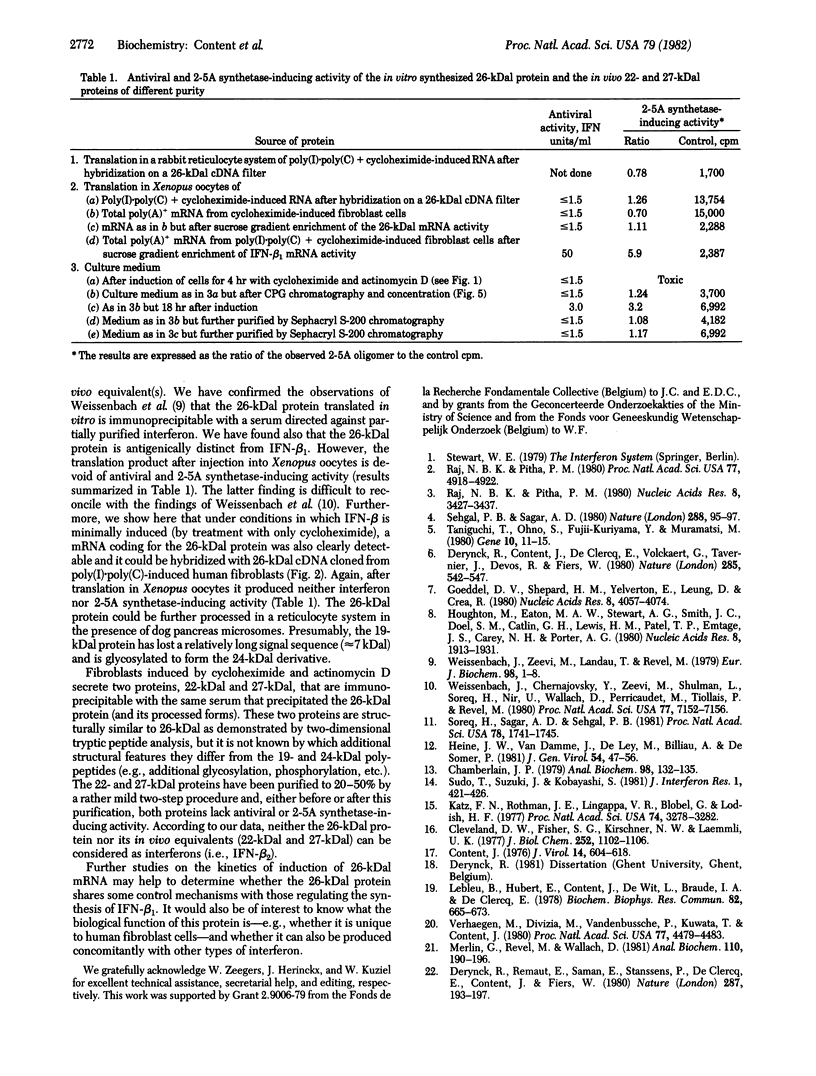
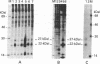Abstract
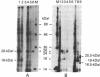
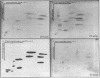
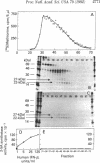
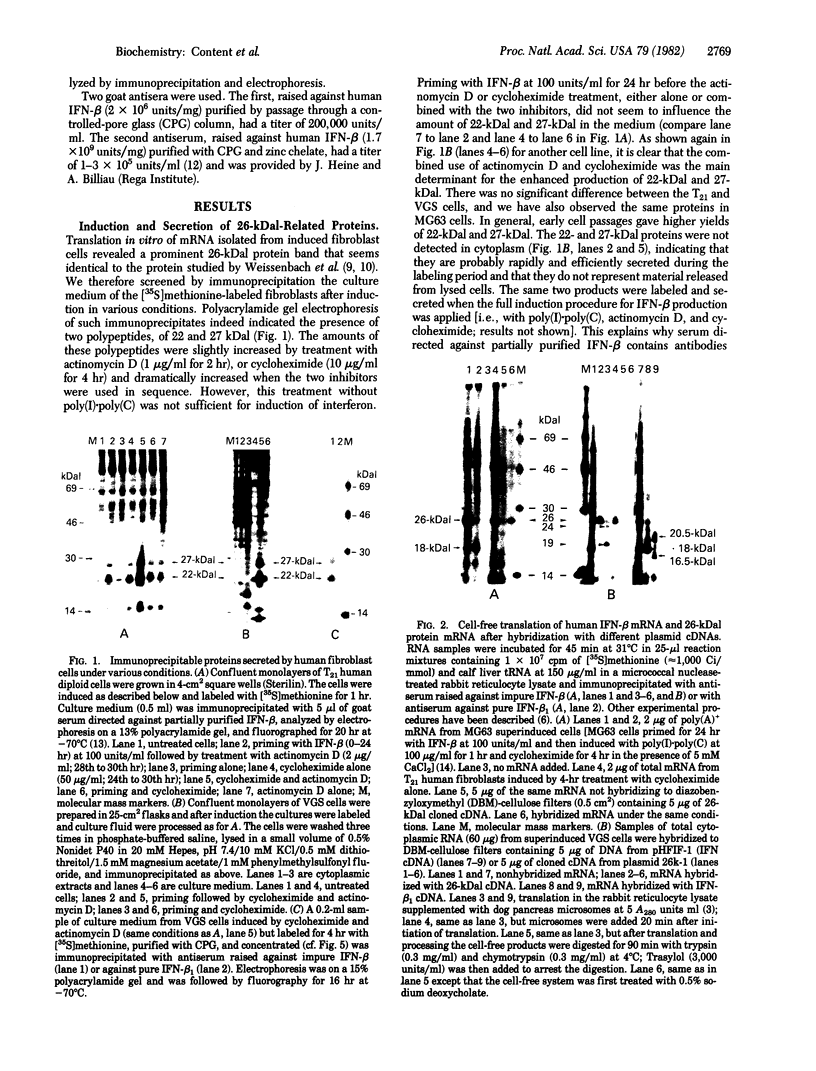
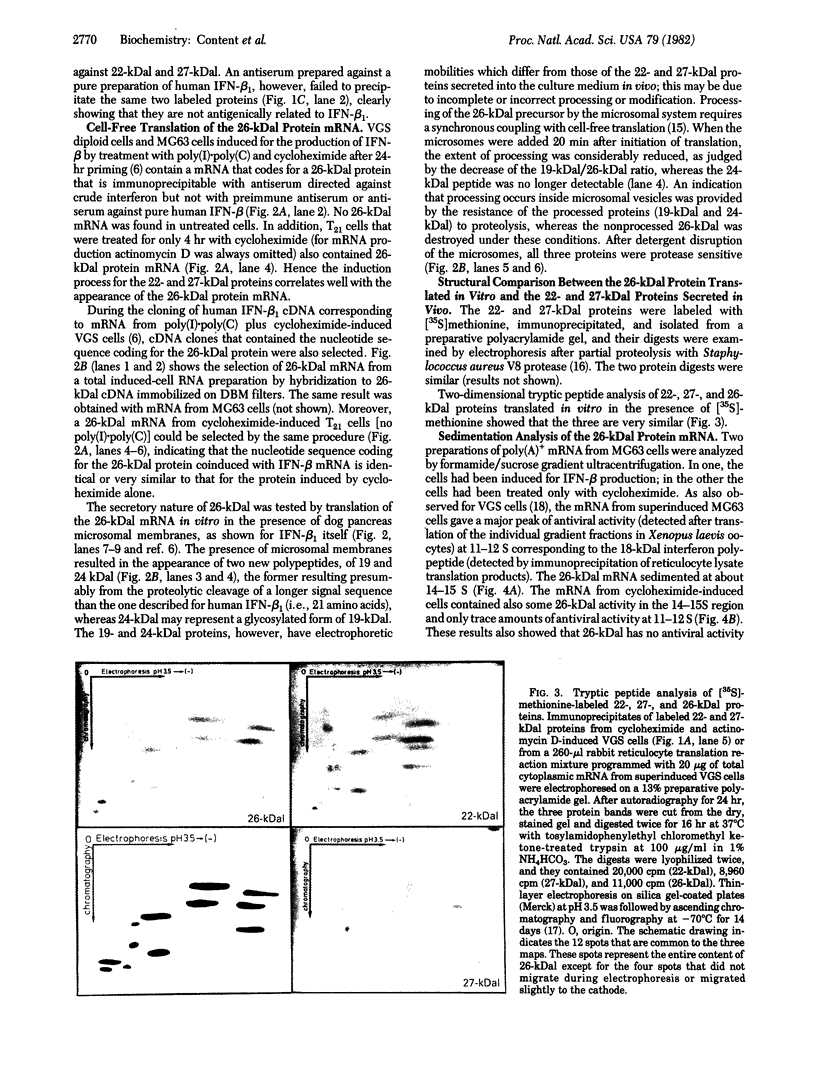
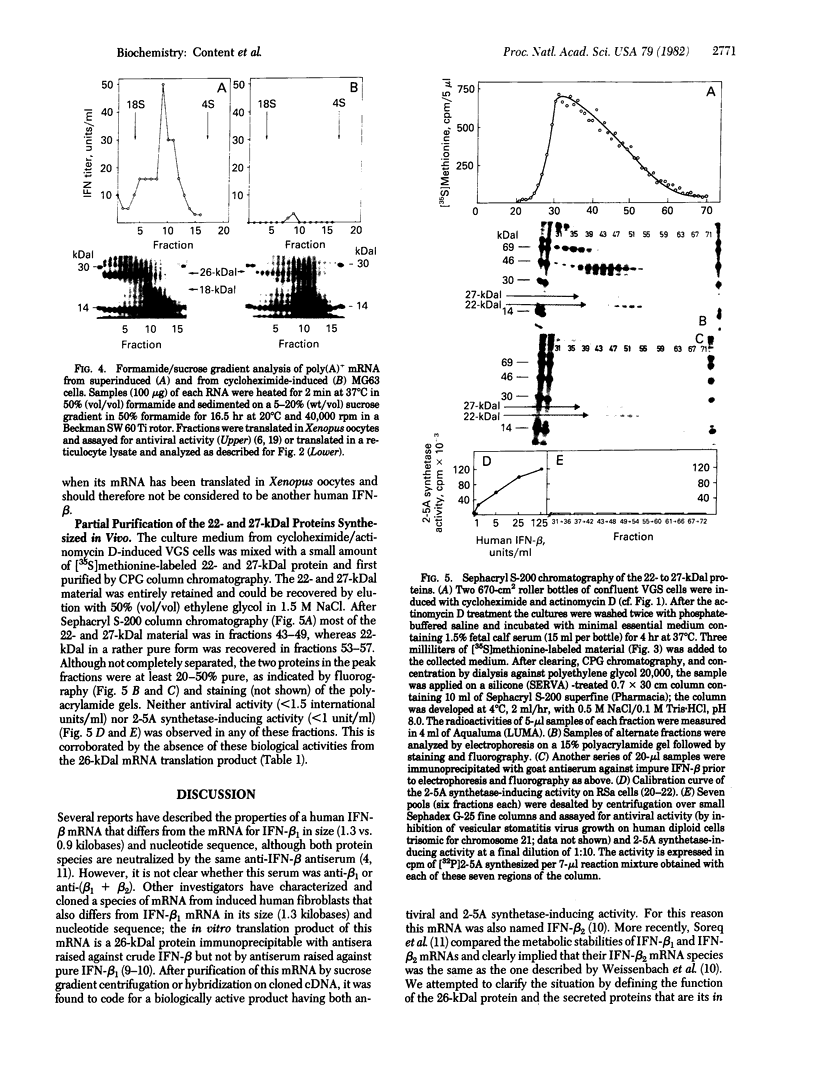
Human fibroblast cells treated with a combination of inhibitors of protein and RNA synthesis [cycloheximide and actinomycin D as used to superinduce interferon beta (IFN-beta)] secrete two proteins with molecular masses of 22000 and 27000 kilodaltons (called 22-kDal and 27-kDal) that are precipitable with an antiserum raised against impure IFN-beta but are antigenically distinct from IFN-beta 1. Translation in vitro of mRNA extracted from human fibroblast cells induced for the production of IFN-beta leads to the synthesis of a 26-kDal protein that is structurally closely related to the 22- and 27-kDal proteins. This 26-kDal protein mRNA is relatively abundant and also appears in human fibroblasts induced only with cycloheximide. It has been partially purified by sucrose gradient centrifugation and more extensively by diazobenzyloxymethyl-cellulose hybridization to plasmid DNA from a bacterial cDNA clone. When translated in an in vitro reticulocyte system supplemented with dog pancreas microsomes, the 26-kDal protein and two other intermediates corresponding presumably to its signal-cleaved (19-kDal) and partially glycosylated (24-kDal) forms were observed. Crude, partially purified, and highly purified 26-kDal mRNA failed to program the synthesis of antiviral or ppp(A2'p5')nA synthetase-inducing activity when translated in Xenopus laevis oocytes. Moreover, partially purified 22-kDal and 27-kDal (i.e., the in vivo equivalents of the 26-kDal protein) are also devoid of antiviral or ppp(A2'p5')nA synthetase-inducing activity. Hence, this 26-kDal mRNA, although presumably identical to the human IFN-beta 2 mRNA described by Weissenbach et al. [Weissenbach, J., Chernajovsky, Y., Zeevi, M., Shulman, K., Soreq, H., Nir. U., Wallach, D., Perricaudet, M., Tiollais, P. & Revel, M. (1980) Proc. Natl. Acad. Sci. USA 77, 7152-7156], cannot be considered to be a fibroblast interferon mRNA.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Content J. Cell-free translation of influenza virus mRNA. J Virol. 1976 May;18(2):604–618. doi: 10.1128/jvi.18.2.604-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Content J., DeClercq E., Volckaert G., Tavernier J., Devos R., Fiers W. Isolation and structure of a human fibroblast interferon gene. Nature. 1980 Jun 19;285(5766):542–547. doi: 10.1038/285542a0. [DOI] [PubMed] [Google Scholar]
- Derynck R., Remaut E., Saman E., Stanssens P., De Clercq E., Content J., Fiers W. Expression of human fibroblast interferon gene in Escherichia coli. Nature. 1980 Sep 18;287(5779):193–197. doi: 10.1038/287193a0. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Shepard H. M., Yelverton E., Leung D., Crea R., Sloma A., Pestka S. Synthesis of human fibroblast interferon by E. coli. Nucleic Acids Res. 1980 Sep 25;8(18):4057–4074. doi: 10.1093/nar/8.18.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Van Damme J., de Ley M., Billiau A., de Somer P. Purification of human fibroblast interferon by zinc chelate chromatography. J Gen Virol. 1981 May;54(Pt 1):47–56. doi: 10.1099/0022-1317-54-1-47. [DOI] [PubMed] [Google Scholar]
- Houghton M., Stewart A. G., Doel S. M., Emtage J. S., Eaton M. A., Smith J. C., Patel T. P., Lewis H. M., Porter A. G., Birch J. R. The amino-terminal sequence of human fibroblast interferon as deduced from reverse transcripts obtained using synthetic oligonucleotide primers. Nucleic Acids Res. 1980 May 10;8(9):1913–1931. doi: 10.1093/nar/8.9.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebleu B., Hubert E., Content J., De Wit L., Braude I. A., De Clercq E. Translation of mouse interferon mRNA in Xenopus laevis oocytes and in rabbit reticulocyte lysates. Biochem Biophys Res Commun. 1978 May 30;82(2):665–673. doi: 10.1016/0006-291x(78)90926-9. [DOI] [PubMed] [Google Scholar]
- Merlin G., Revel M., Wallach D. The interferon-induced enzyme oligo-isoadenylate synthetase: rapid determination of its in vitro products. Anal Biochem. 1981 Jan 1;110(1):190–196. doi: 10.1016/0003-2697(81)90134-2. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Synthesis of new proteins associated with the induction of interferon in human fibroblast cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4918–4922. doi: 10.1073/pnas.77.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. The messenger RNA sequences in human fibroblast cells induced with poly rI.rC to produce interferon. Nucleic Acids Res. 1980 Aug 11;8(15):3427–3437. doi: 10.1093/nar/8.15.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Sagar A. D. Heterogeneity of poly(I) x poly(C)-induced human fibroblast interferon mRNA species. Nature. 1980 Nov 6;288(5786):95–97. doi: 10.1038/288095a0. [DOI] [PubMed] [Google Scholar]
- Soreq H., Sagar A. D., Sehgal P. B. Translational activity and functional stability of human fibroblast beta 1 and beta 2 interferon mRNAs lacking 3'-terminal RNA sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1741–1745. doi: 10.1073/pnas.78.3.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T., Suzuki J., Kobayashi S. Effect of CaCl2 on production of interferon and synthesis of its mRNA in human MG-63 cells. J Interferon Res. 1981;1(3):421–426. doi: 10.1089/jir.1981.1.421. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Ohno S., Fujii-Kuriyama Y., Muramatsu M. The nucleotide sequence of human fibroblast interferon cDNA. Gene. 1980 Jun;10(1):11–15. doi: 10.1016/0378-1119(80)90138-9. [DOI] [PubMed] [Google Scholar]
- Verhaegen M., Divizia M., Vandenbussche P., Kuwata T., Content J. Abnormal behavior of interferon-induced enzymatic activities in an interferon-resistant cell line. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4479–4483. doi: 10.1073/pnas.77.8.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Chernajovsky Y., Zeevi M., Shulman L., Soreq H., Nir U., Wallach D., Perricaudet M., Tiollais P., Revel M. Two interferon mRNAs in human fibroblasts: in vitro translation and Escherichia coli cloning studies. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7152–7156. doi: 10.1073/pnas.77.12.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Zeevi M., Landau T., Revel M. Identification of the translation products of human fibroblast interferon mRNA in reticulocyte lysates. Eur J Biochem. 1979 Jul;98(1):1–8. doi: 10.1111/j.1432-1033.1979.tb13153.x. [DOI] [PubMed] [Google Scholar]