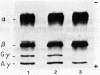
Abstract
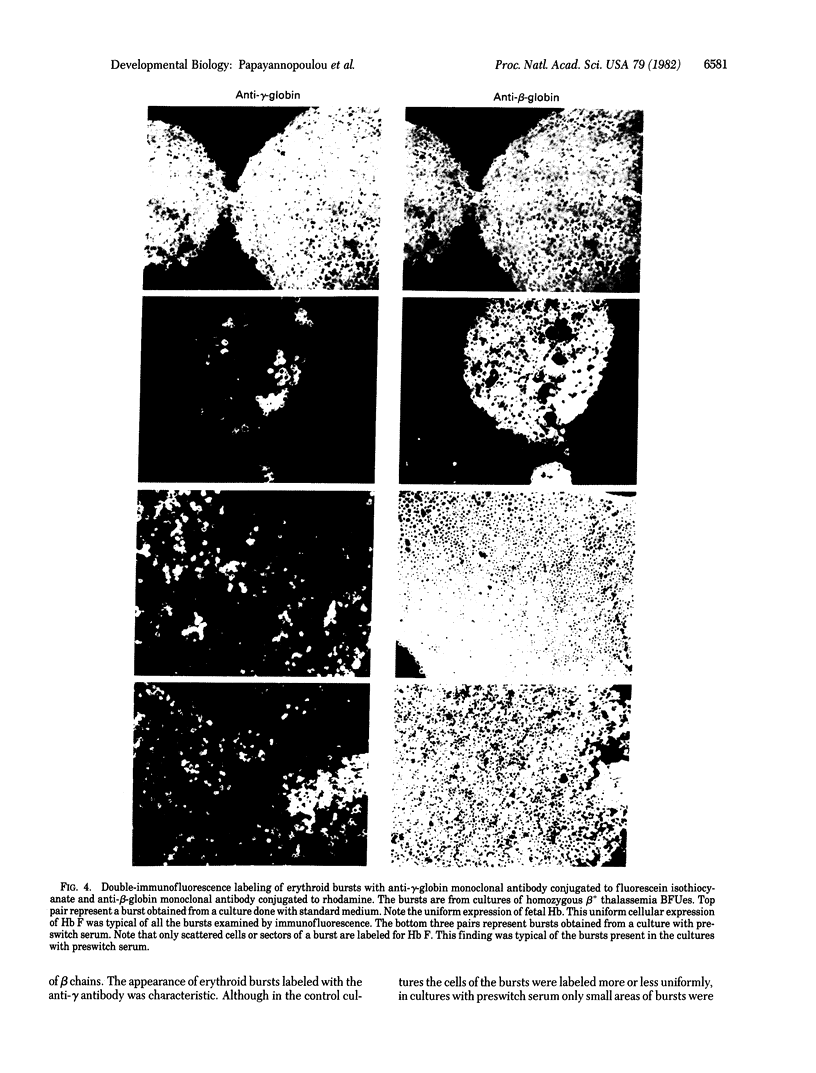
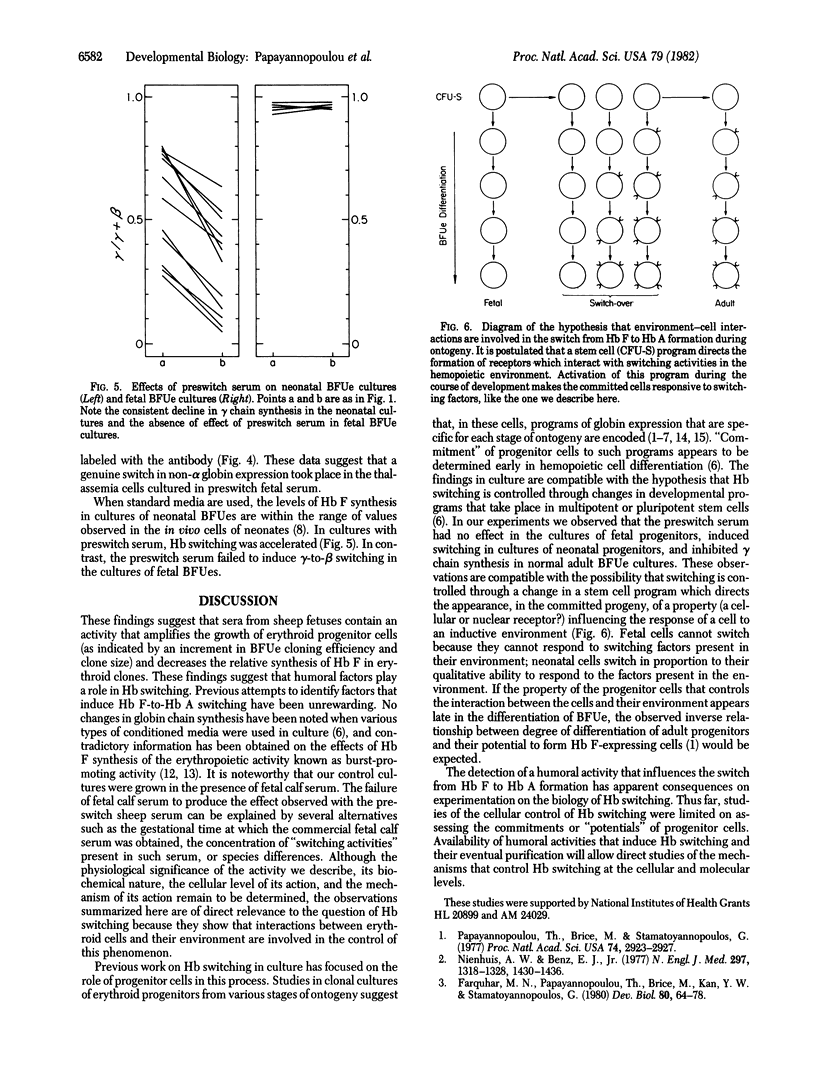
An erythropoietic activity that exerts a profound effect on fetal Hb synthesis is present in fetal sheep sera and it attains a peak concentration at the end of the second to the middle of the third trimester of fetal life. The activity consistently inhibits the increased synthesis of fetal Hb in cultures of burst-forming units (BFUes) from normal adults. In cultures of BFUes from homozygous beta+-thalassemias the activity produces a striking decline in gamma chain synthesis, a decline in G gamma/A gamma chain synthesis ratio, and an increase in delta/gamma and alpha/non-alpha ratios--i.e., findings suggesting a genuine gamma-to-beta switch. The activity accelerates Hb F-to-Hb A switching in neonatal BFUe cultures but it has no effect on fetal Hb synthesis in cultures of BFUe obtained from human fetuses. These findings provide direct evidence that (a) humoral factors play a role in the regulation of the switch from fetal to adult Hb formation, and (b) progenitor cells from various stages of ontogeny respond differently to these factors. The results are compatible with the hypothesis that Hb switching during development is mediated through a change in a developmental program which controls the responsiveness of progenitor cells to "switching" activities in their environment.
Full text
PDF
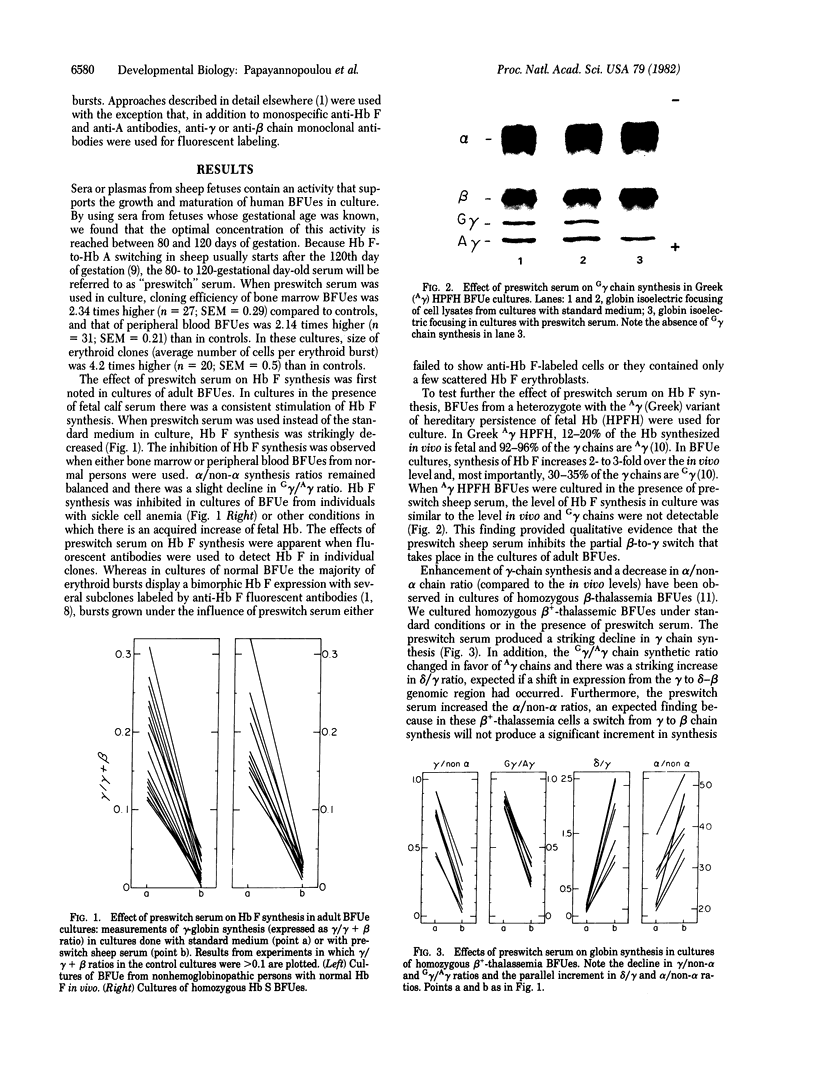

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beuzard Y., Tulliez M., Testa U., Vainchenker W., Dubart A., Tsapis A., Galacteros F., Breton-Gorius J., Rosa J. Beta-thalassemia and sickle cell disease in culture of early erythroid precursors: hemoglobin synthesis and ultrastructural study. Blood Cells. 1981;7(1):179–200. [PubMed] [Google Scholar]
- Darbre P. D., Adamson J. W., Wood W. G., Weatherall D. J., Robinson J. S. Patterns of globin chain synthesis in erythroid colonies grown from sheep marrow of different developmental stages. Br J Haematol. 1979 Apr;41(4):459–475. doi: 10.1111/j.1365-2141.1979.tb05884.x. [DOI] [PubMed] [Google Scholar]
- Dean A., Schechter A. N., Papayannopoulou T., Stamatoyannopoulos G. Heterogeneity of erythroid precursor cells. Hemoglobin quantitation in single clones by radioimmunoassay. J Biol Chem. 1981 Mar 10;256(5):2447–2453. [PubMed] [Google Scholar]
- Farquhar M. N., Papayannopoulou T., Brice M., Kan Y. W., Stamatoyannopoulos G. Cellular regulation of fetal hemoglobin synthesis in man. Investigation of gamma and beta mRNA accumulation in clonal erythroid cultures initiated from erythroid progenitors derived from fetuses, neonates, and adult individuals. Dev Biol. 1980 Nov;80(1):64–78. doi: 10.1016/0012-1606(80)90499-6. [DOI] [PubMed] [Google Scholar]
- Kidoguchi K., Ogawa M., Karam J. D., McNeil J. S., Fitch M. S. Hemoglobin biosynthesis in individual bursts in culture: studies of human umbilical cord blood. Blood. 1979 Mar;53(3):519–522. [PubMed] [Google Scholar]
- Nienhuis A. W., Benz E. J., Jr Regulation of hemoglobin synthesis during the development of the red cell (third of three parts). N Engl J Med. 1977 Dec 29;297(26):1430–1436. doi: 10.1056/NEJM197712292972604. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Lawn R. M., Stamatoyannopoulos G., Maniatis T. Greek (A gamma) variant of hereditary persistence of fetal haemoglobin: globin gene organization and studies of expression of fetal haemoglobins in clonal erythroid cultures. Br J Haematol. 1982 Mar;50(3):387–399. doi: 10.1111/j.1365-2141.1982.tb01934.x. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Kurachi S., Stamatoyannopoulos G. Globin synthesis in erythroid bursts that mature sequentially in culture. I. Studies in cultures of adult peripheral blood BFU-Es. Blood. 1981 Nov;58(5):969–974. [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Rosenblum B. B., Papayannopoulou T., Brice M., Nakamoto B., Shepard T. H. HbF and HbA production in erythroid cultures from human fetuses and neonates. Blood. 1979 Aug;54(2):440–450. [PubMed] [Google Scholar]
- Terasawa T., Ogawa M., Porter P. N., Golde D. W., Goldwasser E. Effect of burst-promoting activity (BPA) and erythropoietin on hemoglobin biosynthesis in culture. Blood. 1980 Dec;56(6):1106–1110. [PubMed] [Google Scholar]
- Testa U., Vainchenker W., Guerrasio A., Beuzard Y., Breton-Gorius J., Rosa J., Lusis A. J., Golde D. Hb switching in neonatal cultures. Increase of Hb A synthesis in presence of an erythroid potentiating activity (EPA). J Cell Physiol. 1982 Feb;110(2):196–202. doi: 10.1002/jcp.1041100214. [DOI] [PubMed] [Google Scholar]