Abstract
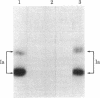
The expression of Ia-like antigens by cultured human endothelial cells has been investigated by means of monoclonal antibody binding to intact cells and by immunoprecipitation of radioiodinated membrane proteins. Primary growing and confluent cultures of human umbilical vein endothelium express little, if any, detectable Ia-like antigens under standard culture conditions. However, treatment of primary cultures with the lectin phytohemagglutinin induces the expression of Ia-like antigens. This action of the lectin uniformly affects all the endothelial cells in a culture, does not depend on cell division, and is associated with a cell shape change. The data presented in this report provide unequivocal serological and biochemical demonstration of Ia-like antigens on human vascular endothelial cells. The fact that the expression of Ia-like antigens by endothelium can be induced may have important implications for organ transplantation and for regulation of the immunological response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashida E. R., Johnson A. R., Lipsky P. E. Human endothelial cell-lymphocyte interaction. Endothelial cells function as accessory cells necessary for mitogen-induced human T lymphocyte activation in vitro. J Clin Invest. 1981 May;67(5):1490–1499. doi: 10.1172/JCI110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin W. M., 3rd, Claas F. H., van Es L. A., van Rood J. J. Distribution of endothelial-monocyte and HLA antigens on renal vascular endothelium. Transplant Proc. 1981 Mar;13(1 Pt 1):103–107. [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Bodmer W. F., Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979 Jul;9(7):536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Bodmer W. F. Monoclonal antibodies to HLA--DRw determinants. Tissue Antigens. 1980 Jul;16(1):30–48. doi: 10.1111/j.1399-0039.1980.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Campbell D. A., Jr, Oehler J. R., Tsai C. T., LoBuglio A. F., Niederhuber J. E. Effect of mouse anti-I region antiserum and complement on human mononuclear cell response to concanavalin A. J Immunol. 1982 May;128(5):2057–2062. [PubMed] [Google Scholar]
- Cohen M. C., Picciano P. T., Douglas W. J., Yoshida T., Kreutzer D. L., Cohen S. Migration inhibition of endothelial cells by lymphokine-containing supernatants. Science. 1982 Jan 15;215(4530):301–303. doi: 10.1126/science.6797069. [DOI] [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Major histocompatibility complex antigens in rat kidney, ureter, and bladder. Localization with monoclonal antibodies and demonstration of Ia-positive dendritic cells. Transplantation. 1981 May;31(5):318–325. doi: 10.1097/00007890-198105010-00003. [DOI] [PubMed] [Google Scholar]
- Hart D. N., Fuggle S. V., Williams K. A., Fabre J. W., Ting A., Morris P. J. Localization of HLA-ABC and DR antigens in human kidney. Transplantation. 1981 Jun;31(6):428–433. doi: 10.1097/00007890-198106000-00005. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Bergh O. J., Thorsby E. Antigen-presenting properties of human vascular endothelial cells. J Exp Med. 1980 Aug 1;152(2 Pt 2):249s–255s. [PubMed] [Google Scholar]
- Hirschberg H., Evensen S. A., Henriksen T., Thorsby E. The human mixed lymphocyte-endothelium culture interaction. Transplantation. 1975 Jun;19(6):495–504. doi: 10.1097/00007890-197506000-00008. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Moen T., Thorsby E. Complement and cell-mediated specific destruction of human endothelial cells treated with anti-DRw antisera. Transplant Proc. 1979 Mar;11(1):776–778. [PubMed] [Google Scholar]
- Hirschberg H. Presentation of viral antigens by human vascular endothelial cells in vitro. Hum Immunol. 1981 May;2(3):235–246. doi: 10.1016/0198-8859(81)90015-x. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Thorsby E. Immunogenicity of foreign tissues. Transplantation. 1981 Jan;31(1):96–97. doi: 10.1097/00007890-198101000-00024. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Humphreys R. E., McCune J. M., Chess L., Herrman H. C., Malenka D. J., Mann D. L., Parham P., Schlossman S. F., Strominger J. L. Isolation and immunologic characterization of a human. B-lymphocyte-specific, cell surface antigen. J Exp Med. 1976 Jul 1;144(1):98–112. doi: 10.1084/jem.144.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häyry P., von Willebrand E., Andersson L. C. Expression of HLA-ABC and -DR locus antigens on human kidney, endothelial, tubular and glomerular cells. Scand J Immunol. 1980;11(3):303–310. doi: 10.1111/j.1365-3083.1980.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Koyama K., Fukunishi T., Barcos M., Tanigaki N., Pressman D. Human Ia-like antigens in non-lymphoid organs. Immunology. 1979 Oct;38(2):333–341. [PMC free article] [PubMed] [Google Scholar]
- Krangel M. S., Orr H. T., Strominger J. L. Assembly and maturation of HLA-A and HLA-B antigens in vivo. Cell. 1979 Dec;18(4):979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moen T., Moen M., Thorsby E. HLA-D region products are expressed in endothelial cells. Tissue Antigens. 1980 Feb;15(2):112–122. doi: 10.1111/j.1399-0039.1980.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Moraes J. R., Stastny P. A new antigen system expressed in human endothelial cells. J Clin Invest. 1977 Aug;60(2):449–454. doi: 10.1172/JCI108795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. J., Williams A. F. Antigens on mouse and rat lymphocytes recognized by rabbit antiserum against rat brain: the quantitative analysis of a xenogeneic antiserum. Eur J Immunol. 1975 Apr;5(4):274–281. doi: 10.1002/eji.1830050412. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Pesando J. M., Yunis E. J., Schlossman S. F. Monoclonal antibodies defining serologically distinct HLA-D/DR related Ia-like antigens in man. Hum Immunol. 1981 Feb;2(1):77–90. doi: 10.1016/0198-8859(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Tomaselli K. J., Yunis E. J., Schlossman S. F., Pesando J. M. Monoclonal antibody identifies a new Ia-like (p29,34) polymorphic system linked to the HLA-D/DR region. Nature. 1981 Apr 16;290(5807):591–593. doi: 10.1038/290591a0. [DOI] [PubMed] [Google Scholar]
- Natali P. G., De Martino C., Quaranta V., Nicotra M. R., Frezza F., Pellegrino M. A., Ferrone S. Expression of Ia-like antigens in normal human nonlymphoid tissues. Transplantation. 1981 Jan;31(1):75–78. doi: 10.1097/00007890-198101000-00017. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Strominger J. L. Rapid purification of detergent-solubilized HLA antigen by affinity chromatography employing anti-beta2-microglobulin serum. J Biol Chem. 1976 Sep 10;251(17):5427–5428. [PubMed] [Google Scholar]
- Scher M. G., Beller D. I., Unanue E. R. Demonstration of a soluble mediator that induces exudates rich in Ia-positive macrophages. J Exp Med. 1980 Dec 1;152(6):1684–1698. doi: 10.1084/jem.152.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Kiely J. M., Cotran R. S., Unanue E. R. Characterization of resident glomerular cells in the rat expressing Ia determinants and manifesting genetically restricted interactions with lymphocytes. J Clin Invest. 1981 Oct;68(4):920–931. doi: 10.1172/JCI110347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. A., Mann D. L., van Rood J. J., Ferrara G. B., Strominger J. L. Human B-cell alloantigens DC1, MT1, and LB12 are identical to each other but distinct from the HLA-DR antigen. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4566–4570. doi: 10.1073/pnas.78.7.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Oppenheim J. J. Regulation of murine macrophage Ia-antigen expression by products of activated spleen cells. J Exp Med. 1980 Dec 1;152(6):1734–1744. doi: 10.1084/jem.152.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Nogueira N., Witmer M. D., Tydings J. D., Mellman I. S. Lymphokine enhances the expression and synthesis of Ia antigens on cultured mouse peritoneal macrophages. J Exp Med. 1980 Nov 1;152(5):1248–1261. doi: 10.1084/jem.152.5.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Overlin V., Severson C. D., Parsons T. J., Herbick J., Strauss R. G., Burns C. P., Claas F. H. Demonstration of granulocyte, monocyte, and endothelial cell antigens by double fluorochromatic microcytotoxicity testing. Transplant Proc. 1980 Sep;12(3 Suppl 1):26–31. [PubMed] [Google Scholar]