Abstract
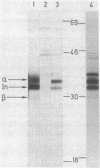
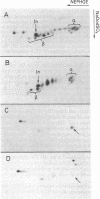
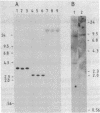
HLA-DR antigens, the human equivalent of mouse Ia antigens, are multimeric surface glycoproteins characterized by a high degree of allelic polymorphism. They are expressed specifically on macrophages and lymphocytes and they play a key role in the regulation of the immune response. We have investigated this complex genetic system by a direct study of the genes involved through molecular cloning. This paper deals with the cloning, in plasmids, of full-length cDNA sequences for the HLA-DR alpha chain from the human B-cell line Raji. The approach relies on a translation assay of mRNA injected into frog oocytes and recognition of translation products by polyclonal and monoclonal antibodies. After enrichment of specific mRNA and cloning of cDNA, plasmid clones were analyzed by hybridization-selection of mRNA and translation in oocytes. A clone was identified and used to screen a cDNA library from which several full-length HLA-DR alpha chain plasmids were isolated. DNA sequence determination of one such clone confirmed its identity and also established the amino acid sequence of the NH2-terminal signal sequence of HLA-DR alpha chains. The translation product of HLA-DR alpha chain mRNA purified by hybridization-selection gives a single alpha chain spot on two-dimensional gels, whereas the alpha chain released from the alpha/beta HLA-DR complex gives about seven distinct spots. Finally, the results of analysis of genomic DNA by Southern blotting are compatible with the existence of a single nonpolymorphic alpha chain gene and indicate extensive cross-hybridization with a homologous gene in mouse DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accolla R. S., Gross N., Carrel S., Corte G. Distinct forms of both alpha and beta subunits are present in the human Ia molecular pool. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4549–4551. doi: 10.1073/pnas.78.7.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselbergs F. A. Post-synthetic fate of the translation products of messenger RNA microinjected into Xenopus oocytes. Mol Biol Rep. 1979 Dec 31;5(4):199–208. doi: 10.1007/BF00782889. [DOI] [PubMed] [Google Scholar]
- Carrel S., Tosi R., Gross N., Tanigaki N., Carmagnola A. L., Accolla R. S. Subsets of human Ia-like molecules defined by monoclonal antibodies. Mol Immunol. 1981 May;18(5):403–411. doi: 10.1016/0161-5890(81)90102-4. [DOI] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Analysis of HLA-D region-associated molecules with monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6567–6571. doi: 10.1073/pnas.76.12.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Knudsen P. J., Kaufman J. F., Strominger J. L. cDNA clones for the heavy chain of HLA-DR antigens obtained after immunopurification of polysomes by monoclonal antibody. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1844–1848. doi: 10.1073/pnas.79.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman A. J., Ploegh H. L., Kaufman J. F., Owen M. J., Strominger J. L. Cell-free synthesis and processing of the heavy and light chains of HLA-DR antigens. J Exp Med. 1980 Aug 1;152(2 Pt 2):65s–82s. [PubMed] [Google Scholar]
- Lee J. S., Trowsdale J., Bodmer W. F. cDNA clones coding for the heavy chain of human HLA-DR antigen. Proc Natl Acad Sci U S A. 1982 Jan;79(2):545–549. doi: 10.1073/pnas.79.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Gross N., Wake C. T., Mach J. P., Carrel S., Accolla R., Mach B. Translation and assembly of HLA-DR antigens in Xenopus oocytes injected with mRNA from a human B-cell line. EMBO J. 1982;1(5):649–654. doi: 10.1002/j.1460-2075.1982.tb01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Kourilsky P., Mach B. Insertion of a rabbit beta-globin gene sequence into an E. coli plasmid. Nucleic Acids Res. 1975 Dec;2(12):2365–2378. doi: 10.1093/nar/2.12.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. A., Strominger J. L. Demonstration of structural polymorphism among HLA-DR light chains by two-dimensional gel electrophoresis. J Exp Med. 1980 Jan 1;151(1):144–165. doi: 10.1084/jem.151.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Ferrone S. Structural polymorphism of human DR antigens. Nature. 1979 May 31;279(5712):436–437. doi: 10.1038/279436a0. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. 3'-end labeling of DNA with [alpha-32P]cordycepin-5'-triphosphate. Gene. 1980 Jul;10(2):177–183. doi: 10.1016/0378-1119(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W., Ryffel G. U., Wyler T., Jaggi F. B., Weber R., Dawid I. B. Cloning and characterization of synthetic sequences from the Xenopus iaevis vitellogenin structural gene. Dev Biol. 1978 Dec;67(2):371–383. doi: 10.1016/0012-1606(78)90207-5. [DOI] [PubMed] [Google Scholar]