Abstract
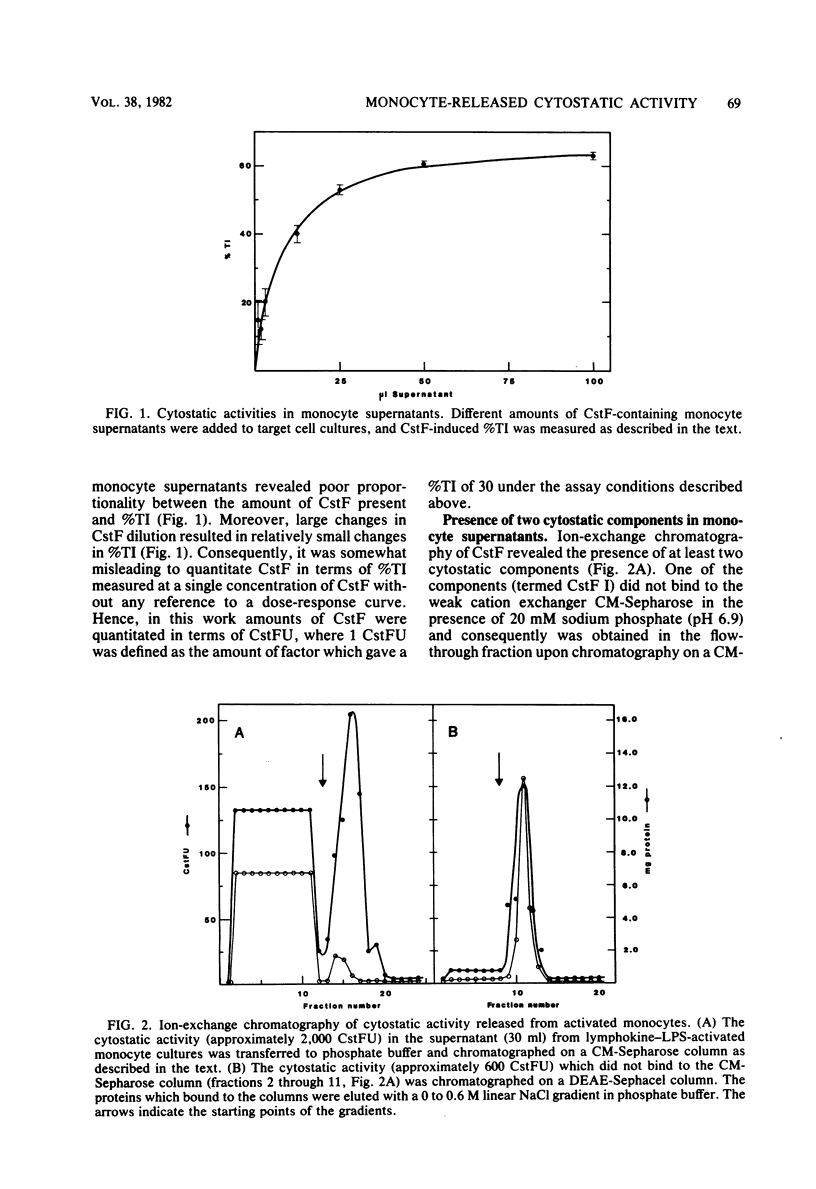
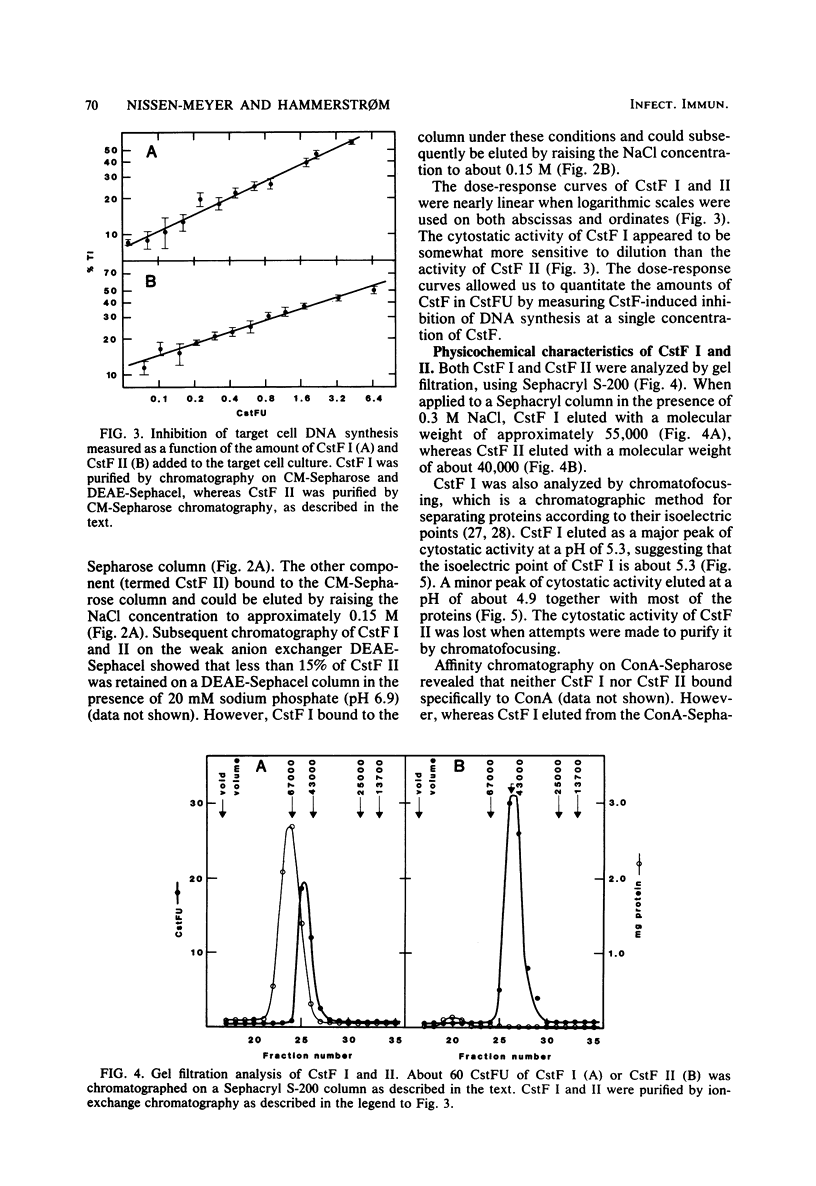
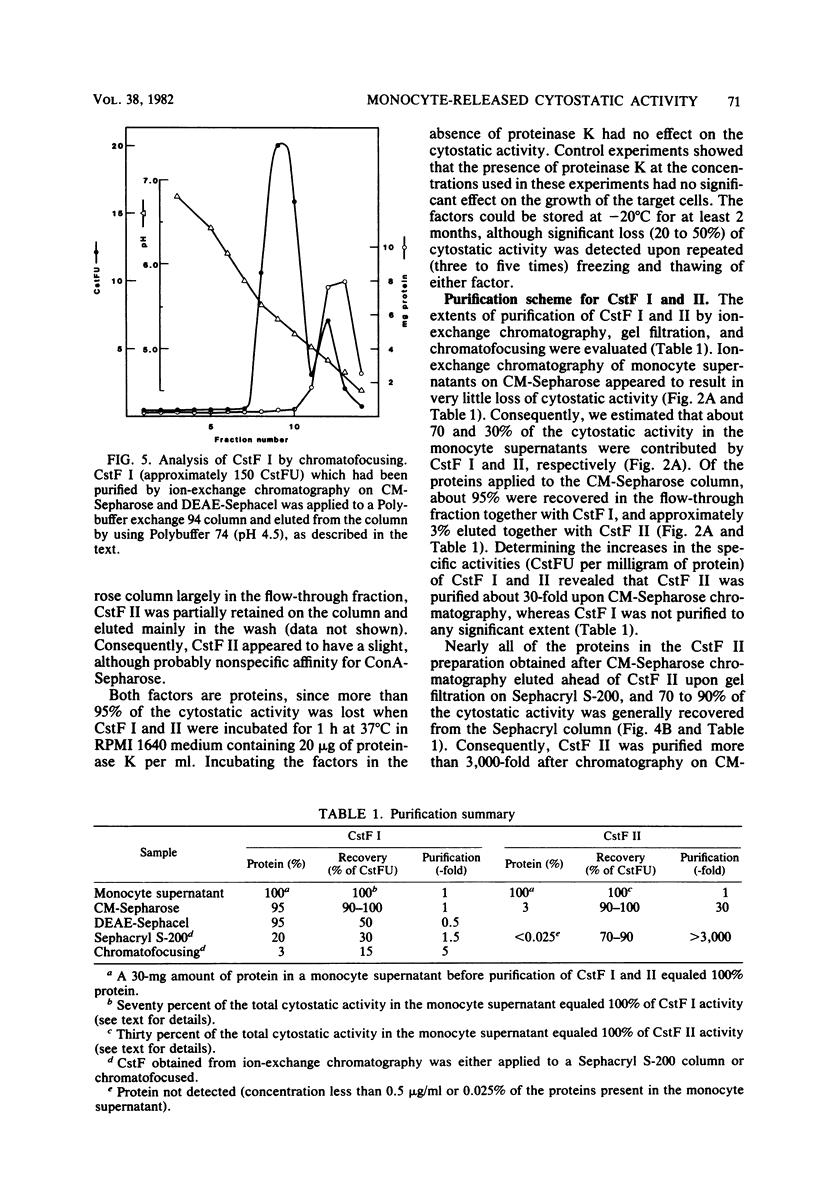
Cultured human monocytes released cytostatic activity upon in vitro activation with lymphokines and lipopolysaccharide. This activity was mainly due to the presence of two different cytostatic factors, termed CstF I and II, which were separated by ion-exchange chromatography. At neutral pH, CstF I bound to the weak anion exchanger DEAE-Sephacel but not to the weak cation exchanger CM-Sepharose, whereas CstF II bound to CM-Sepharose but not to DEAE-Sephacel. The molecular weights of CstF I and II as determined by gel filtration were 55,000 and 40,000, respectively. Upon chromatofocusing, CstF I behaved as if it had an isoelectric point of 5.3. Neither CstF I nor CstF II bound specifically to concanavalin A-Sepharose, indicating the absence of carbohydrate residues containing alpha-D-mannopyranosyl, alpha-D-glucopyranosyl, or sterically related components. Both factors were susceptible to inactivation by proteinase K, demonstrating their protein nature. CstF II was purified more than 3,000-fold upon chromatography on CM-Sepharose and Sephacryl S-200. Ion-exchange chromatography and chromatofocusing of CstF I removed 97% of the proteins in the monocyte supernatant, but only 15% of the activity was recovered, resulting in a fivefold purification of CstF I.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. Effector mechanisms of cytolytically activated macrophages. I. Secretion of neutral proteases and effect of protease inhibitors. J Immunol. 1980 Jan;124(1):286–292. [PubMed] [Google Scholar]
- Adams D. O., Johnson W. J., Fiorito E., Nathan C. F. Hydrogen peroxide and cytolytic factor can interact synergistically in effecting cytolysis of neoplastic targets. J Immunol. 1981 Nov;127(5):1973–1977. [PubMed] [Google Scholar]
- Adams D. O., Kao K. J., Farb R., Pizzo S. V. Effector mechanisms of cytolytically activated macrophages. II. Secretion of a cytolytic factor by activated macrophages and its relationship to secreted neutral proteases. J Immunol. 1980 Jan;124(1):293–300. [PubMed] [Google Scholar]
- Adams D. O., Snyderman R. Do macrophages destroy nascent tumors? J Natl Cancer Inst. 1979 Jun;62(6):1341–1345. [PubMed] [Google Scholar]
- Alexander P. The functions of the macrophage in malignant disease. Annu Rev Med. 1976;27:207–224. doi: 10.1146/annurev.me.27.020176.001231. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Hammerström J., Unsgaard G., Lamvik J. Activation of human monocytes by mediators from lymphocytes stimulated with Corynebacterium parvum. Acta Pathol Microbiol Scand C. 1979 Jun;87C(3):167–175. [PubMed] [Google Scholar]
- Hammerstrøm J. Human macrophage differentiation in vivo and in vitro. A comparison of human peritoneal macrophages and monocytes. Acta Pathol Microbiol Scand C. 1979 Apr;87C(2):113–120. [PubMed] [Google Scholar]
- Hammerstrøm J. Human monocyte-mediated cytotoxicity to K-562 cells: activation by lymphokines. Scand J Immunol. 1979;10(6):575–584. doi: 10.1111/j.1365-3083.1979.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Hammerstrøm J. In vitro influence of endotoxin on human mononuclear phagocyte structure and function. 2. Enhancement of the expression of cytoststic and cytolytic activity of normal and lymphokine-activated monocytes. Acta Pathol Microbiol Scand C. 1979 Dec;87(6):391–399. [PubMed] [Google Scholar]
- Hammestrøm J. Soluble cytostatic factor(s) released from human monocytes. I. Production and effect on normal and transformed human target cells. Scand J Immunol. 1982 Mar;15(3):311–318. doi: 10.1111/j.1365-3083.1982.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Discrimination between neoplastic and non-neoplastic cells in vitro by activated macrophages. J Natl Cancer Inst. 1974 Nov;53(5):1487–1492. doi: 10.1093/jnci/53.5.1487. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Matthews N. Production of an anti-tumour cytotoxin by human monocytes. Immunology. 1981 Sep;44(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Ryley H. C., Neale M. L. Tumour-necrosis factor from the rabbit. IV. Purification and chemical characterization. Br J Cancer. 1980 Sep;42(3):416–422. doi: 10.1038/bjc.1980.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N. Tumour-necrosis factor from the rabbit. II. Production by monocytes. Br J Cancer. 1978 Aug;38(2):310–315. doi: 10.1038/bjc.1978.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Watkins J. F. Tumour-necrosis factor from the rabbit. I. Mode of action, specificity and physicochemical properties. Br J Cancer. 1978 Aug;38(2):302–309. doi: 10.1038/bjc.1978.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Falk W., Meltzer M. S. Inhibition of nonspecific tumoricidal activity by activated macrophages with antiserum against a soluble cytotoxic factor. Infect Immun. 1981 Jul;33(1):156–164. doi: 10.1128/iai.33.1.156-164.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Meltzer M. S., Mergenhagen S. E. Generation and characterization of a lipopolysaccharide-induced and serum-derived cytotoxic factor for tumor cells. Infect Immun. 1980 Apr;28(1):204–211. doi: 10.1128/iai.28.1.204-211.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Moore R. N., Mergenhagen S. E. Macrophages as a source of tumoricidal activity (tumor-necrotizing factor). Infect Immun. 1980 Nov;30(2):523–530. doi: 10.1128/iai.30.2.523-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Root R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med. 1977 Dec 1;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piessens W. F., Churchill W. H., Jr, David Macrophages activated in vitro with lymphocyte mediators kill neoplastic but not normal cells. J Immunol. 1975 Jan;114(1 Pt 2):293–299. [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Purification and physico-chemical characterization of rabbit tumor necrosis factor. J Immunol. 1980 Oct;125(4):1671–1677. [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Rabbit tumor necrosis factor: mechanism of action. Infect Immun. 1981 Jan;31(1):380–385. doi: 10.1128/iai.31.1.380-385.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


