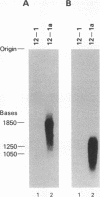
Abstract
Murine teratocarcinoma stem cells, unlike most other cell types, do not express major histocompatibility antigens. The steady-state levels of beta 2-microglobulin and H-2 mRNA from F9-derived teratocarcinoma stem and differentiated cells were examined by blot hybridization using cloned DNA probes specific for these mRNAs. No H-2- or beta 2-microglobulin-specific RNA was detected in F9 teratocarcinoma stem cells (clone 12-1); thus, F9 teratocarcinoma stem cells (clone 12-1) contain no more than 1/10 the H-2 and beta 2-microglobulin mRNAs of the differentiated daughter cells (clone 12-1a). We suggest that this regulation of major histocompatibility antigen expression is due to transcriptional control of the major histocompatibility antigen genes, H-2 and beta 2-microglobulin. The transcriptional regulation of these genes is accompanied by a change in their DNase I sensitivity. Normally, transcriptionally inactive genes are DNase I resistant, while active genes are DNase I sensitive. In contrast, the silent major histocompatibility antigen genes of teratocarcinoma stem cells are more DNase I sensitive than the active genes of the differentiated cells.
Full text
PDF
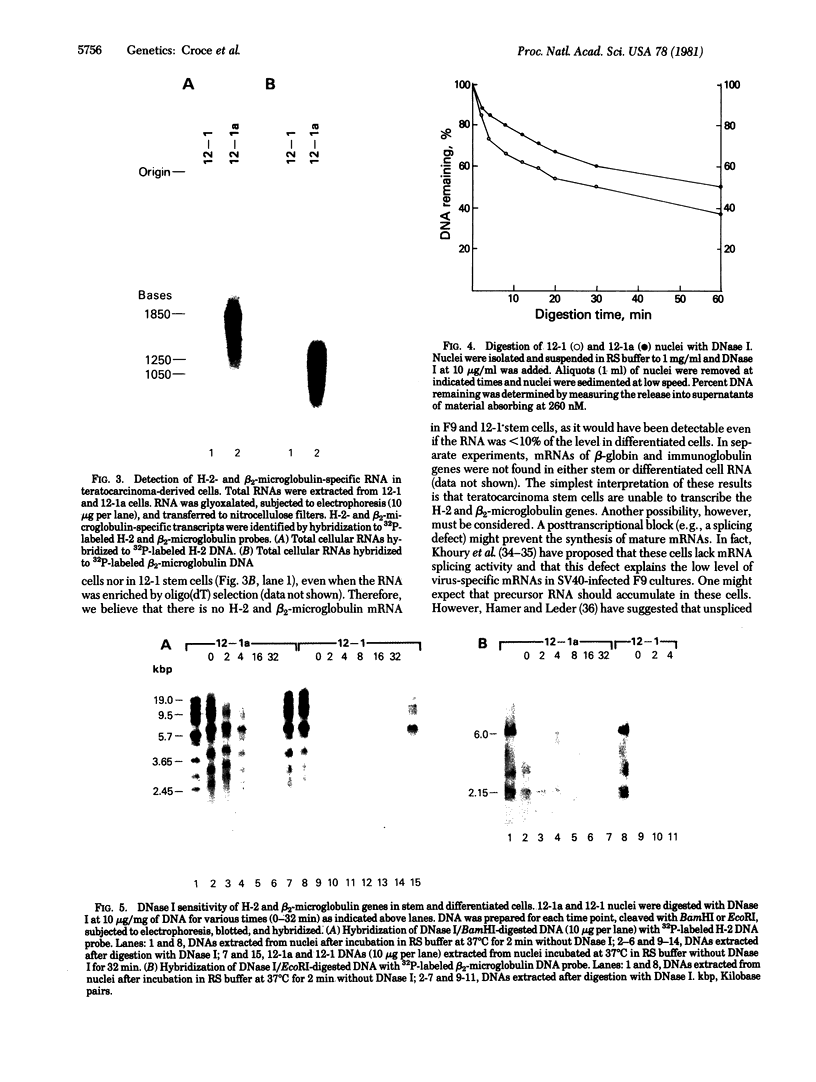


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artzt K., Jacob F. Letter: Absence of serologically detectable H-2 on primitive teratocarcinoma cells in culture. Transplantation. 1974 Jun;17(6):632–634. doi: 10.1097/00007890-197406000-00015. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Berstine E. G., Hooper M. L., Grandchamp S., Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breindl M., Bacheler L., Fan H., Jaenisch R. Chromatin conformation of integrated Moloney leukemia virus DNA sequences in tissues of BALB/Mo mice and in virus-infected cell lines. J Virol. 1980 May;34(2):373–382. doi: 10.1128/jvi.34.2.373-382.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrin P. E., Nilsson K. Beta 2-microglobulin production in vitro by human hematopoietic, mesenchymal, and epithelial cells. J Immunol. 1974 Jan;112(1):137–144. [PubMed] [Google Scholar]
- Flint S. J., Weintraub H. M. An altered subunit configuration associated with the actively transcribed DNA of integrated adenovirus genes. Cell. 1977 Nov;12(3):783–794. doi: 10.1016/0092-8674(77)90277-x. [DOI] [PubMed] [Google Scholar]
- Forman J., Vitetta E. S. Absence of H-2 antigens capable of reacting with cytotoxic T cells on a teratoma line expressing a T/t locus antigen. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3661–3665. doi: 10.1073/pnas.72.9.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gmür R., Solter D., Knowles B. B. Independent regulation of H-2K and H-2D gene expression in murine teratocarcinoma somatic cell hybrids. J Exp Med. 1980 Jun 1;151(6):1349–1359. doi: 10.1084/jem.151.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Das S., Neiman P., Weintraub H. Regulation of expression and chromosomal subunit conformation of avian retrovirus genomes. Cell. 1978 Aug;14(4):865–878. doi: 10.1016/0092-8674(78)90342-2. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Splicing and the formation of stable RNA. Cell. 1979 Dec;18(4):1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- Henriksen O., Robinson E. A., Appella E. Purification and chemical characterization of papain-solubilized histocompatibility-2 antigens from mouse liver. J Biol Chem. 1979 Aug 25;254(16):7651–7658. [PubMed] [Google Scholar]
- Heyner S., Brinster R. L., Palm J. Effect of iso-antibody on pre-implantation mouse embryos. Nature. 1969 May 24;222(5195):783–784. doi: 10.1038/222783a0. [DOI] [PubMed] [Google Scholar]
- Heyner S. Detection of H-2 antigens on the cells of the early mouse embryo. Transplantation. 1973 Dec;16(6):675–678. [PubMed] [Google Scholar]
- Huebner K., Linnenbach A., Weidner S., Glenn G., Croce C. M. Deoxyribonuclease I sensitivity of plasmid genomes in teratocarcinoma-derived stem and differentiated cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5071–5075. doi: 10.1073/pnas.78.8.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E., Sherman M. I. Stimulation of differentiation of several murine embryonal carcinoma cell lines by retinoic acid. Exp Cell Res. 1979 Dec;124(2):381–391. doi: 10.1016/0014-4827(79)90213-1. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Pan S., Solter D., Linnenbach A., Croce C., Huebner K. Expression of H-2, laminin and SV40 T and TASA on differentiation of transformed murine teratocarcinoma cells. Nature. 1980 Dec 11;288(5791):615–618. doi: 10.1038/288615a0. [DOI] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Linnenbach A., Huebner K., Croce C. M. DNA-transformed murine teratocarcinoma cells: regulation of expression of simian virus 40 tumor antigen in stem versus differentiated cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4875–4879. doi: 10.1073/pnas.77.8.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas J. F., Dubois P., Jakob H., Gaillard J., Jacob F. Tératocarcinome de la souris: différenciation en culture d'une lignée de cellules primitives a potentialités multiples. Ann Microbiol (Paris) 1975 Jan;126(1):3–22. [PubMed] [Google Scholar]
- Palm J., Heyner S., Brinster R. L. Differential immunofluorescence of fertilized mouse eggs with H-2 and non-H-2 antibody. J Exp Med. 1971 Jun 1;133(6):1282–1293. doi: 10.1084/jem.133.6.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthey H., Edidin M. Evidence for the time of appearance of H-2 antigens in mouse development. Transplantation. 1973 Feb;15(2):211–214. doi: 10.1097/00007890-197302000-00004. [DOI] [PubMed] [Google Scholar]
- Segal S., Khoury G. Differentiation as a requirement for simian virus 40 gene expression in F-9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5611–5615. doi: 10.1073/pnas.76.11.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S., Levine A. J., Khoury G. Evidence for non-spliced SV40 RNA in undifferentiated murine teratocarcinoma stem cells. Nature. 1979 Jul 26;280(5720):335–338. doi: 10.1038/280335a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. The arrangement and rearrangement of antibody genes. Nature. 1978 Dec 21;276(5690):790–795. doi: 10.1038/276790a0. [DOI] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Developmental stage-specific antigens during mouse embryogenesis. Curr Top Dev Biol. 1979;13(Pt 1):139–165. doi: 10.1016/s0070-2153(08)60693-6. [DOI] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter D., Shevinsky L., Knowles B. B., Strickland S. The induction of antigenic changes in a teratocarcinoma stem cell line (F9) by retinoic acid. Dev Biol. 1979 Jun;70(2):515–521. doi: 10.1016/0012-1606(79)90043-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]