Abstract
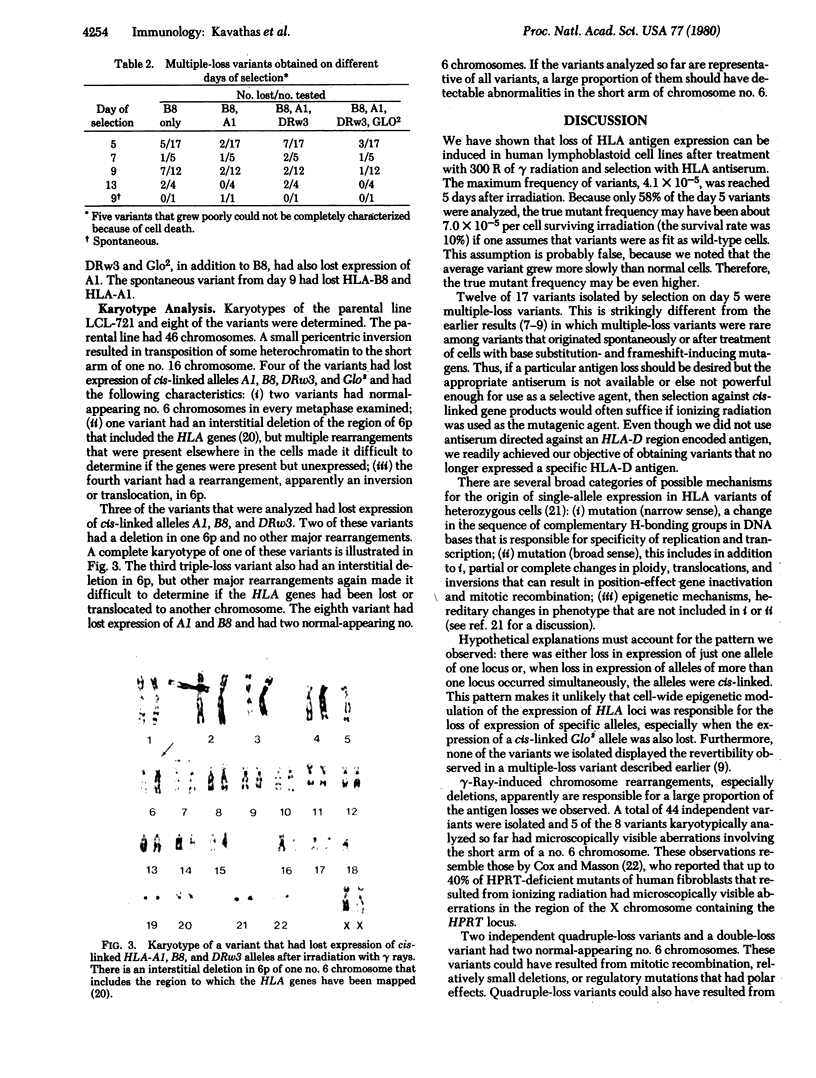
Gamma rays from a cesium source were used to generate human lymphoblastoid cell line variants that had lost expression of all major histocompatibility complex antigens coded for by a single haplotype. The cell line was heterozygous at the glyoxalase I locus and had the HLA haplotypes HLA-A1, B8, DRw3, and HLA-A2, B5, DRw1. We selected with anti-HLA-B8 antiserum in a population of cells that had been irradiated with 300 R. The incidence of B8-loss variants was 4.1 X 10(-5) on day 5 after irradiation. Analysis of variants showed that expressions of HLA and GLO alleles trans to B8 were retained. However, expression of additional cis-linked HLA and GLO gene products was lost in 12 of 17 variants. Variants that had lost expression of (i) HLA-B8, (ii) HLA-B8, A1, (iii) HLA-B8, A1, DRw3, or (iv) HLA-B8, A1, DRw3 and the cis-linked glyoxalase I allele were obtained. Karyotype analysis was performed on eight variants that had lost expression of two or more cis-linked alleles. Three variants had two normal appearing no. 6 chromosomes, four variants had a deletion that included the region coding for HLA genes on the short arm of one no. 6 chromosome, and one variant had an inversion or translocation involving the short arm of one no. 6.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson S., Wolff S. Re-analysis of radiation-induced specific locus mutations in the mouse. Nature. 1976 Dec 23;264(5588):715–719. doi: 10.1038/264715a0. [DOI] [PubMed] [Google Scholar]
- Berger R., Bernheim A., Sasportes M., Hauptmann G., Hors J., Legrand L., Fellous M. Regional mapping of the HLA on the short arm of chromosome 6. Clin Genet. 1979 Mar;15(3):245–251. doi: 10.1111/j.1399-0004.1979.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Bjaring B., Klein G. Antigenic characterization of heterozygous mouse lymphomas after immunoselection in vivo. J Natl Cancer Inst. 1968 Dec;41(6):1411–1429. [PubMed] [Google Scholar]
- Cox R., Masson W. K. Do radiation-induced thioguanine-resistant mutants of cultured mammalian cells arise by HGPRT gene mutation or X-chromosome rearrangement? Nature. 1978 Dec 7;276(5688):629–630. doi: 10.1038/276629a0. [DOI] [PubMed] [Google Scholar]
- DeMars R., Jackson J. L. Mutagenicity detection with human cells. J Environ Pathol Toxicol. 1977 Nov-Dec;1(2):55–77. [PubMed] [Google Scholar]
- Dickinson W. J., Carson H. L. Regulation of the tissue specificity of an enzyme by a cis-acting genetic element: evidence from interspecific Drosophila hybrids. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4559–4562. doi: 10.1073/pnas.76.9.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrone S., Pellegrino M. A., Reisfeld R. A. A rapid method for direct HL-A typing of cultured lymphoid cells. J Immunol. 1971 Aug;107(2):613–615. [PubMed] [Google Scholar]
- KLEIN G., KLEIN E. Histocompatibility changes in tumors. J Cell Physiol Suppl. 1958 Dec;52(Suppl 1):125–168. doi: 10.1002/jcp.1030520409. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kömpf J., Bissbort S., Gussmann S., Ritter H. Polymorphism of red cell glyoxalase I (EI: 4.4.1.5); a new genetic marker in man. Investigation of 169 mother-child combinations. Humangenetik. 1975;27(2):141–143. doi: 10.1007/BF00273329. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Levy R., Grumet F. C., Ness D., Pious D. Production in vitro of murine antibody to a human histocompatibility alloantigen. Nature. 1978 Feb 2;271(5644):461–462. doi: 10.1038/271461a0. [DOI] [PubMed] [Google Scholar]
- Papermaster B. W., Herzenberg L. A. Isolation and characterization of an isoantigenic variant from a heterozygous mouse lymphoma in culture. J Cell Physiol. 1966 Jun;67(3):407–420. doi: 10.1002/jcp.1040670306. [DOI] [PubMed] [Google Scholar]
- Parr C. W., Bagster I. A., Welch S. G. Human red cell glyoxalase I polymorphism. Biochem Genet. 1977 Feb;15(1-2):109–113. doi: 10.1007/BF00484553. [DOI] [PubMed] [Google Scholar]
- Pious D., Bodmer J., Bodmer W. Antigenic expression and cross reactions in HL-A variants of lymphoid cell lines. Tissue Antigens. 1974;4(3):247–256. doi: 10.1111/j.1399-0039.1974.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Pious D., Hawley P., Forrest G. Isolation and characterization of HL-A variants in cultured human lymphoid cells. Proc Natl Acad Sci U S A. 1973 May;70(5):1397–1400. doi: 10.1073/pnas.70.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pious D., Soderland C. HLA variants of cultured human lymphoid cells: evidence for mutational origin and estimation of mutation rate. Science. 1977 Aug 19;197(4305):769–771. doi: 10.1126/science.70075. [DOI] [PubMed] [Google Scholar]
- Reinsmoen N., Yunis E. J., Bach F. H., Bach M. L. Lymphoblastoid cell lines of homozygous typing cells used for sensitization in PLT. Tissue Antigens. 1977 Jan;9(1):11–16. doi: 10.1111/j.1399-0039.1977.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Sugden B., Mark W. Clonal transformation of adult human leukocytes by Epstein-Barr virus. J Virol. 1977 Sep;23(3):503–508. doi: 10.1128/jvi.23.3.503-508.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki P. I., Bernoco D., Park M. S., Ozturk G., Iwaki Y. Microdroplet testing for HLA-A, -B, -C, and -D antigens. The Phillip Levine Award Lecture. Am J Clin Pathol. 1978 Feb;69(2):103–120. doi: 10.1093/ajcp/69.2.103. [DOI] [PubMed] [Google Scholar]