Abstract
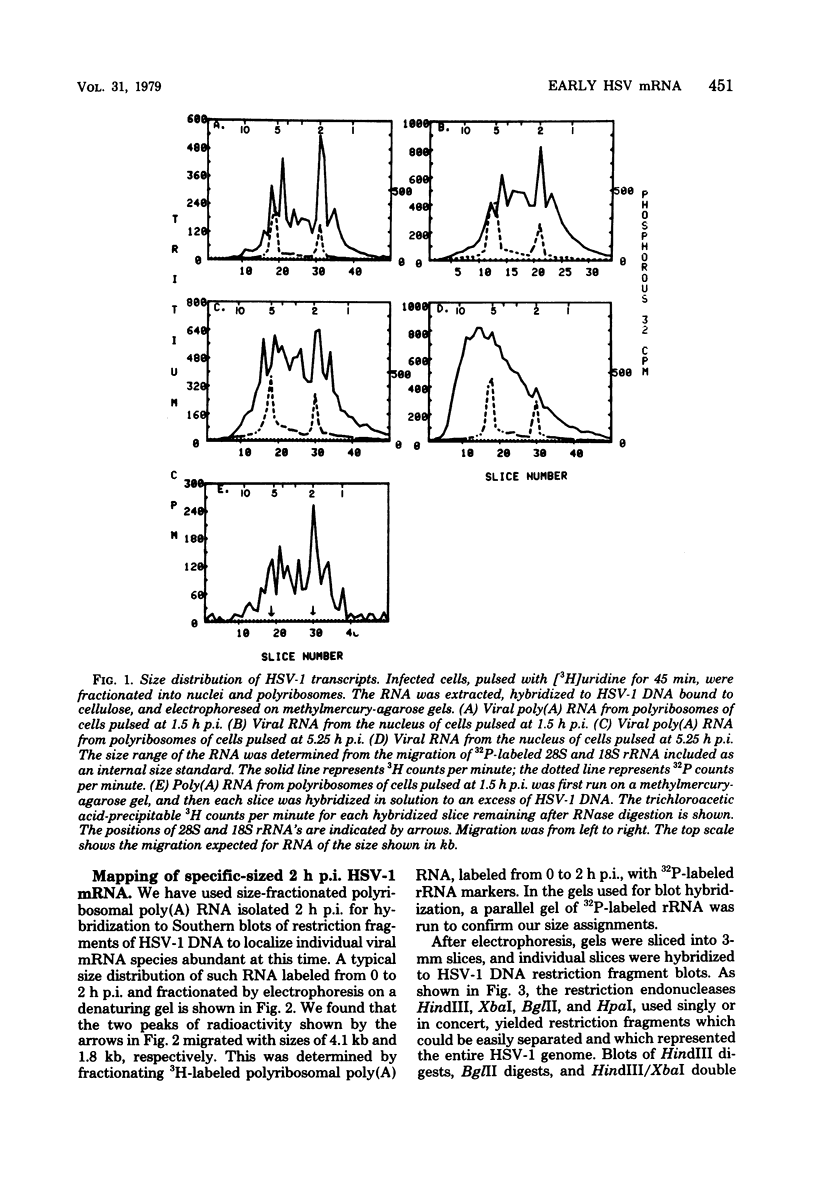
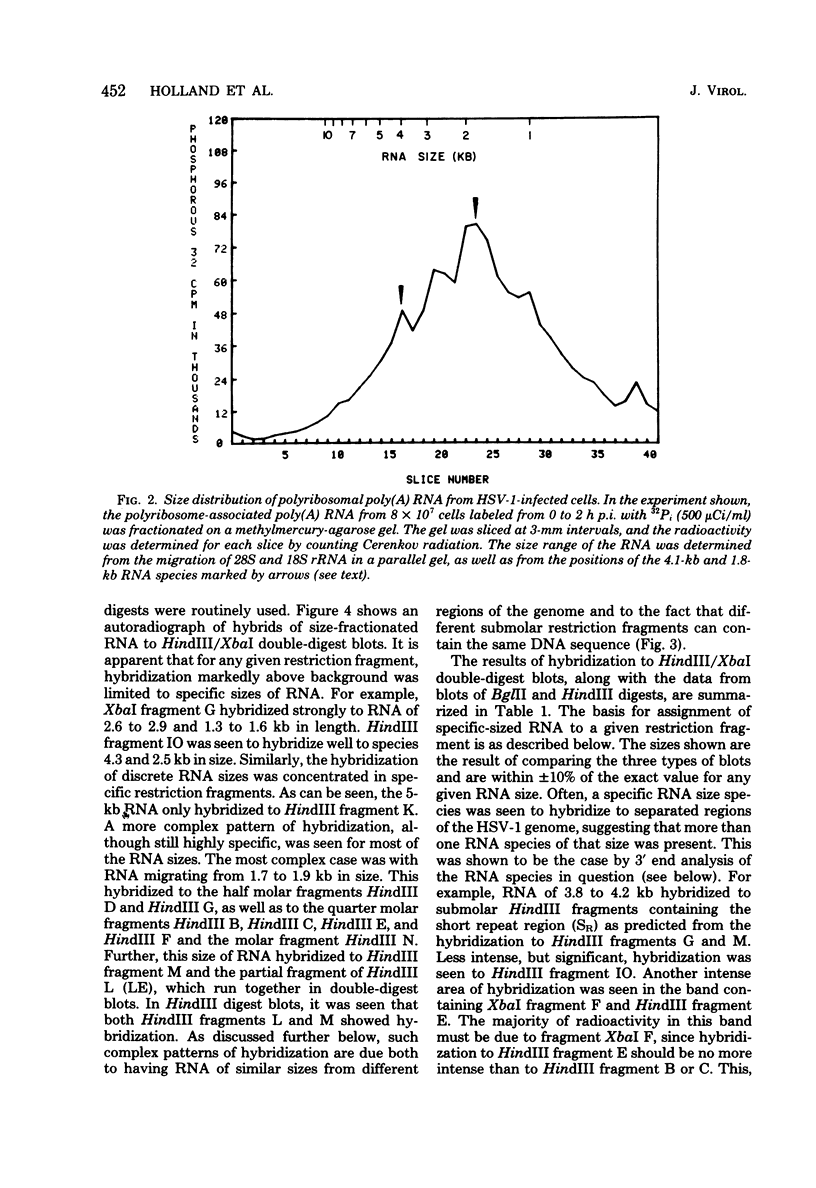
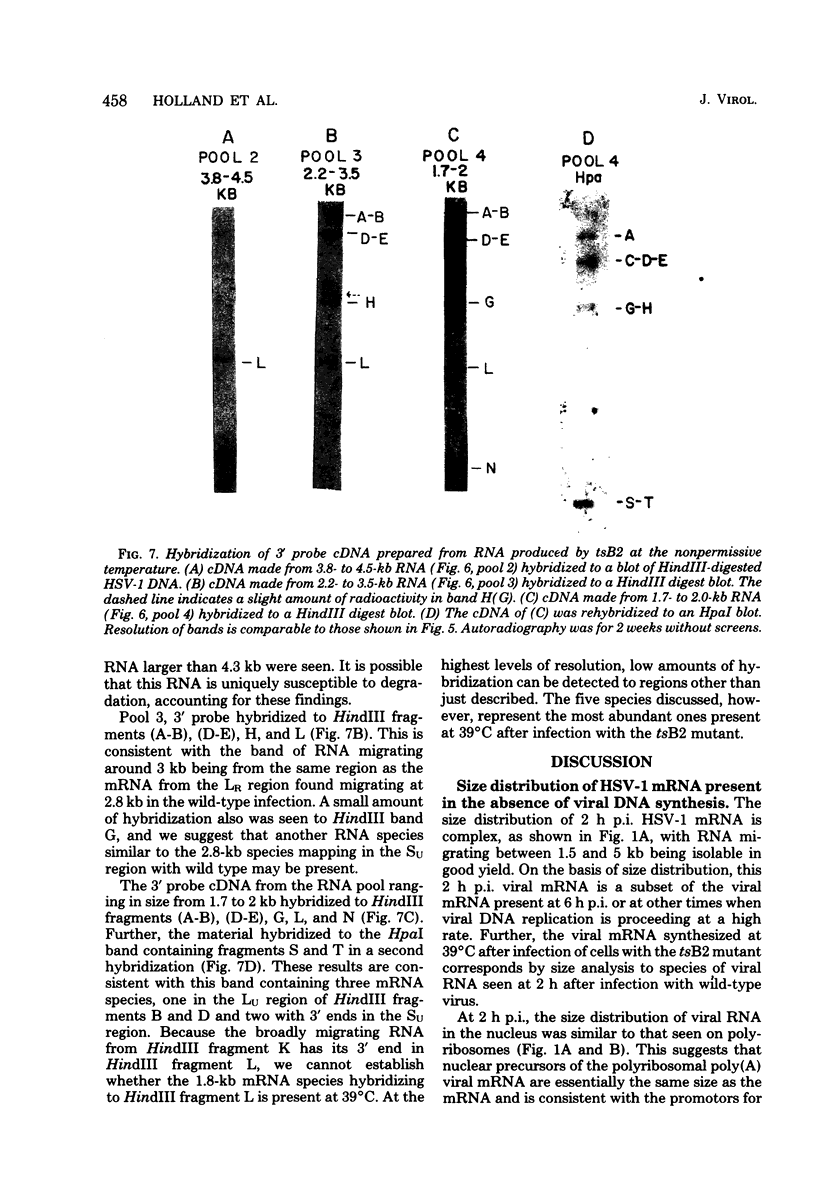
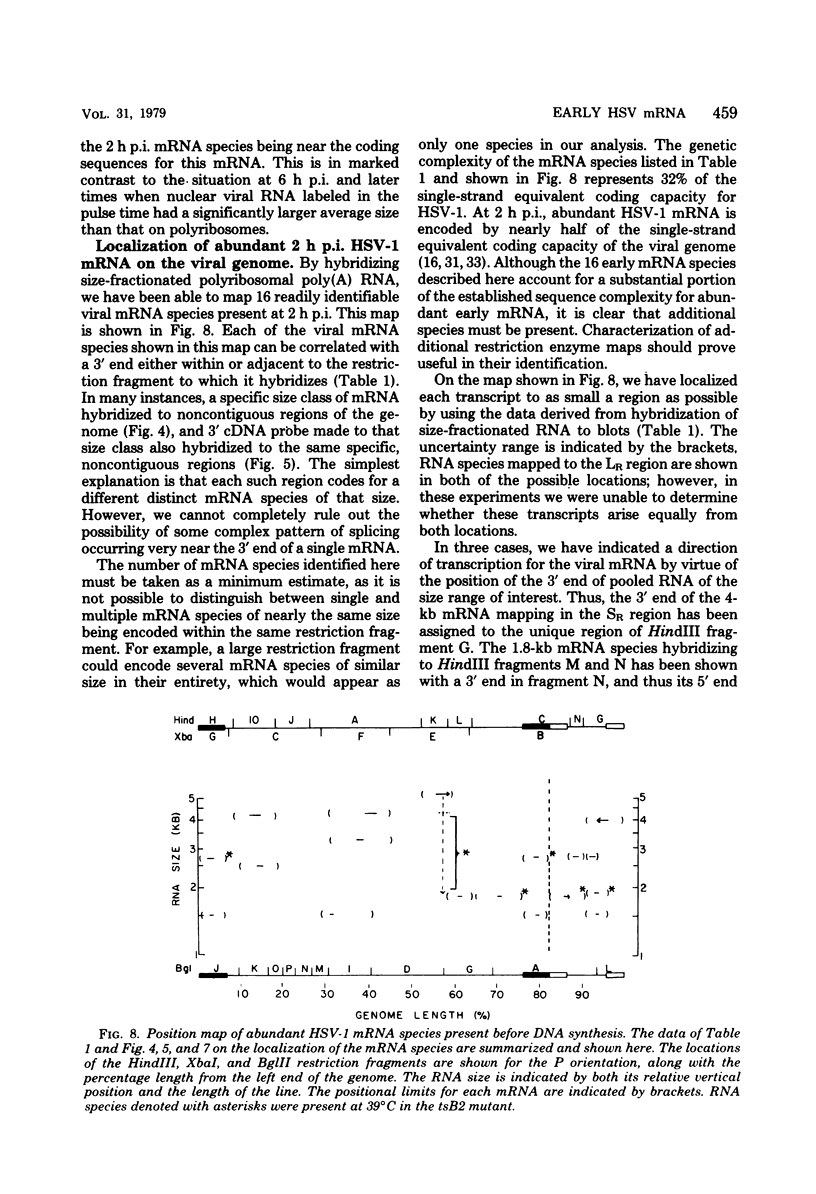
Herpes simplex virus type 1 (HSV-1) DNA covalently bound to cellulose was used as a reagent to isolate viral RNA transcripts for size analysis on denaturing agarose gels. Nuclear and polyribosomal RNA isolated at 2 h postinfection (p.i.) migrated with sizes between 1,500 and 5,500 nucleotides. At 6 h p.i. (when viral DNA synthesis is underway), viral polyribosome-associated polyadenylated RNA showed different discrete sizes of species predominating, with RNA larger than 5,500 nucleotides clearly present. Nearly 50% of the newly made viral RNA found in the nucleus at 6 h p.i. was from 5,000 to 10,000 nucleotides in length. A high-resolution transcription map of the viral mRNA abundant at 2 h p.i. was compiled from the hybridization of Southern blots of HSV-1 DNA restriction fragments to both sizes of fractionated polyribosomal polyadenylated RNA and 3' complementary DNA probe made to this size of fractionated RNA. We have identified and mapped 16 mRNA species abundant at 2 h p.i. These RNAs range in size from 1,500 to 5,300 nucleotides and map throughout the HSV-1 genome. In some instances, a direction of transcription can be suggested. Further, about one-third of this number of mRNA's has been found in cells infected with a DNA-negative temperature-sensitive mutant (tsB2) and grown at the nonpermissive temperature (39 degrees C).
Full text
PDF




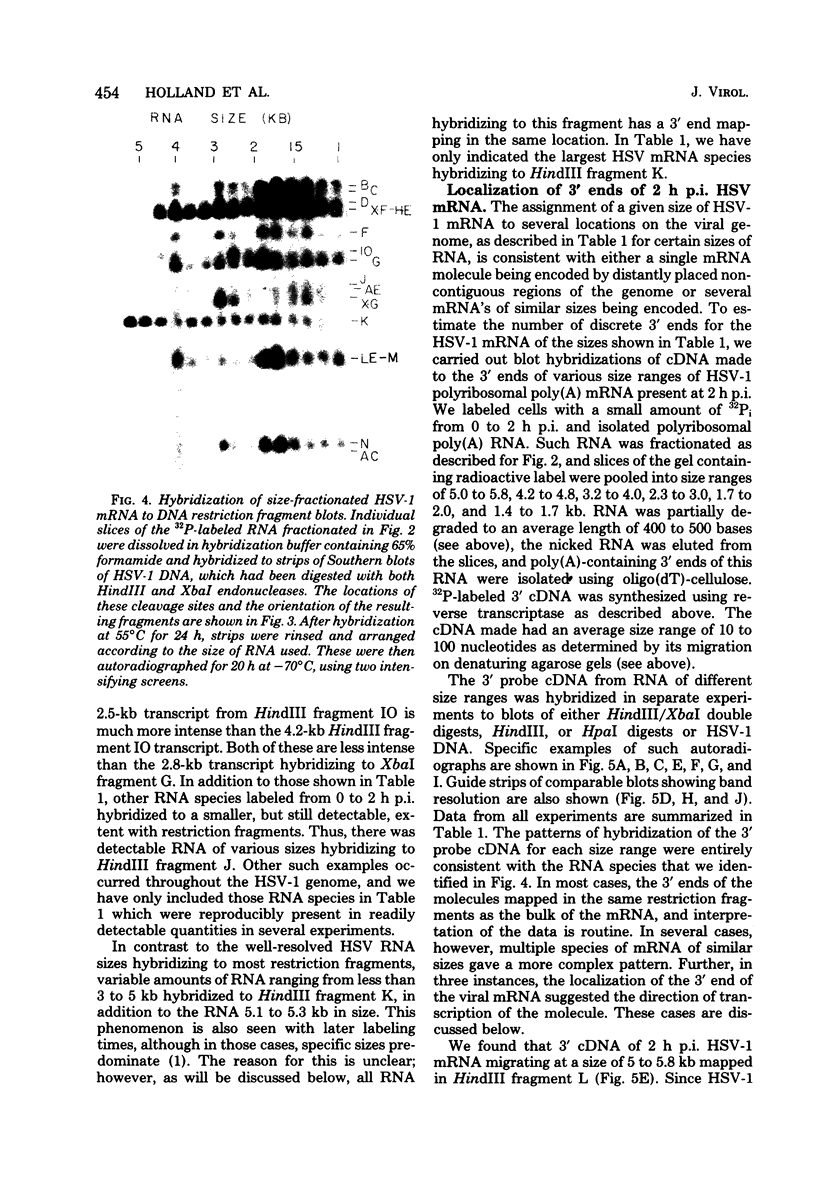
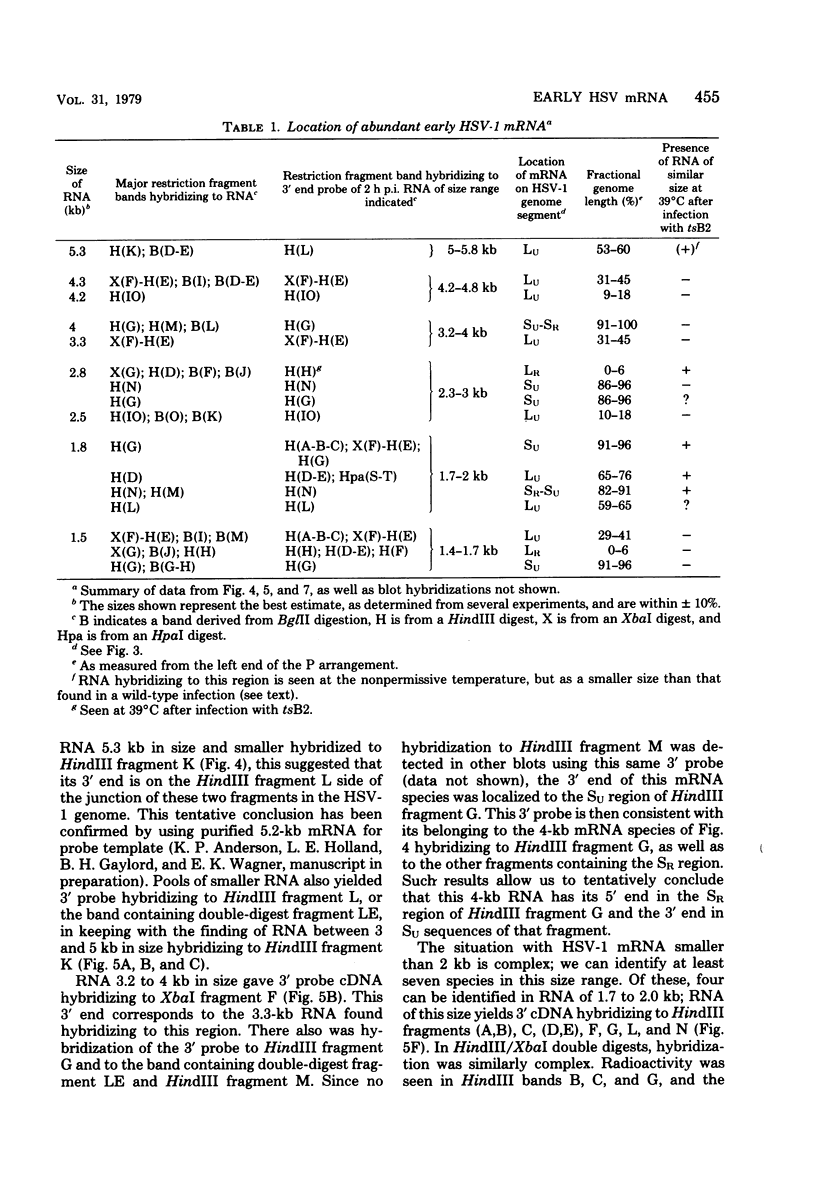
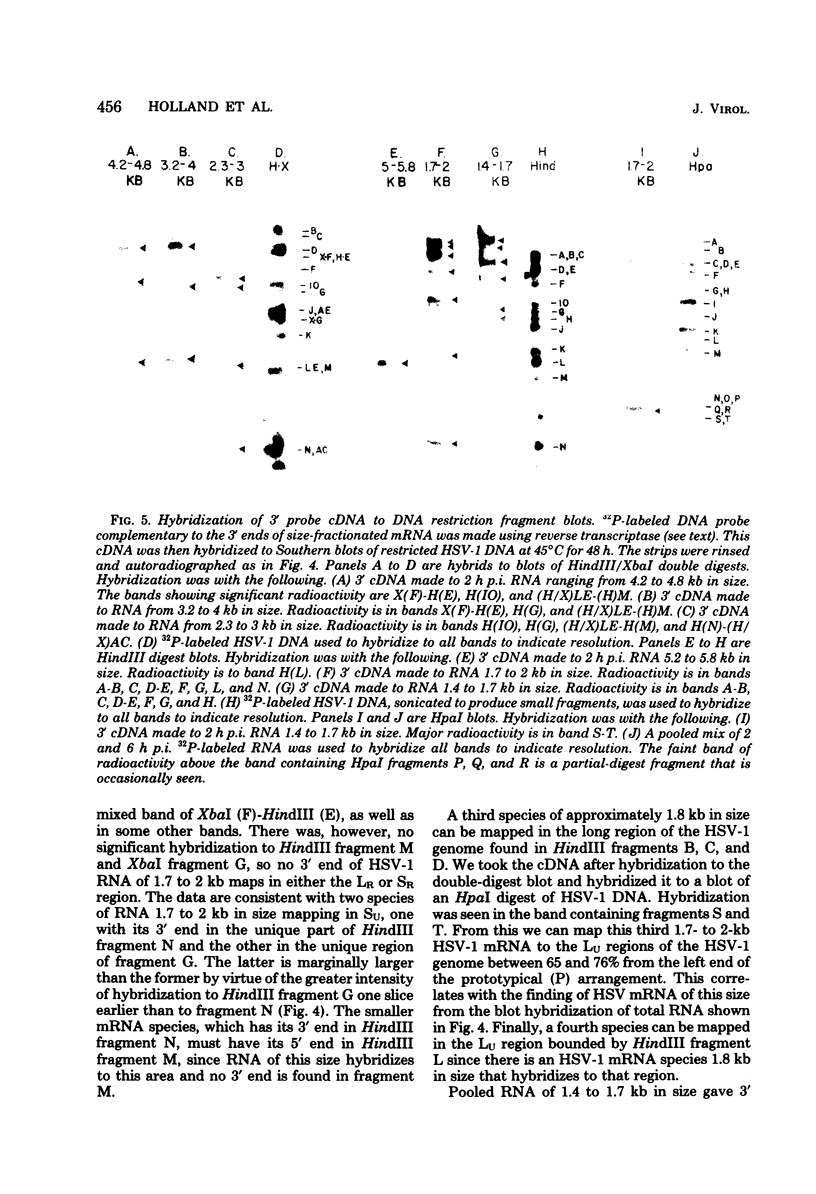
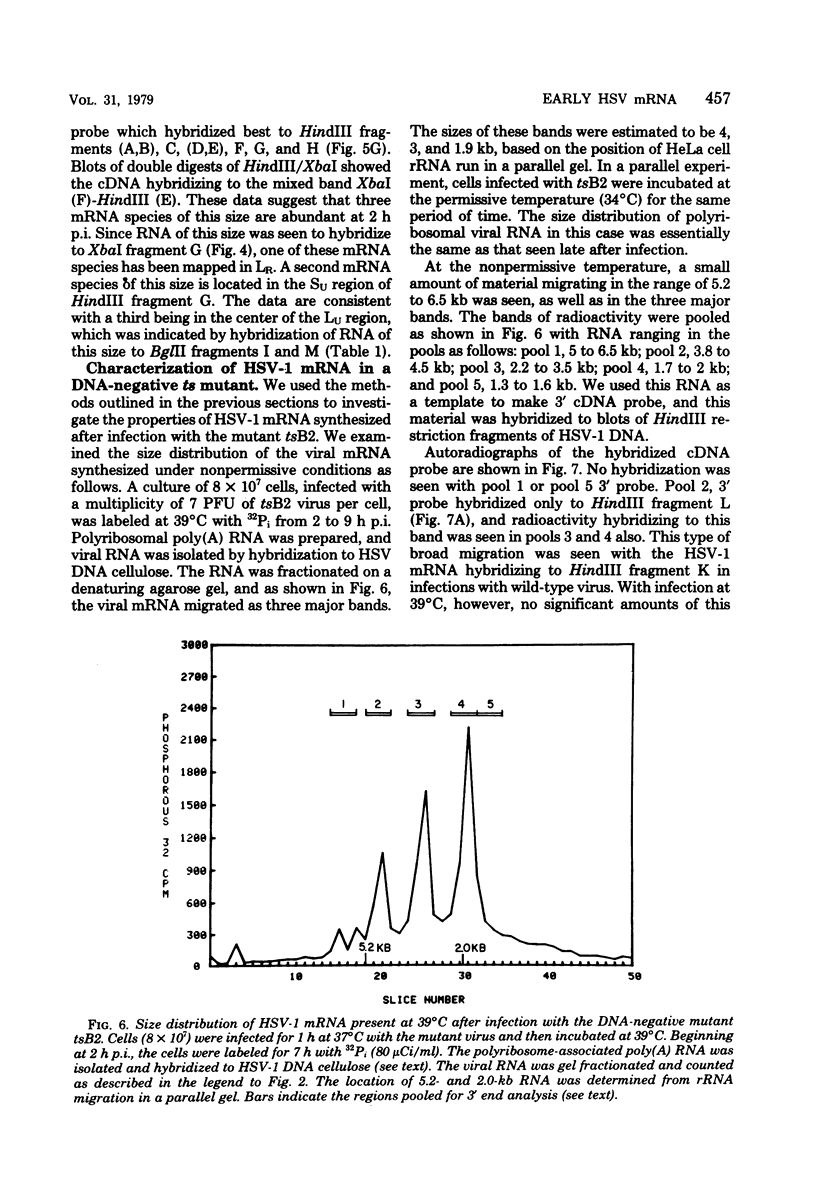



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. P., Stringer J. R., Holland L. E., Wagner E. K. Isolation and localization of herpes simplex virus type 1 mRNA. J Virol. 1979 Jun;30(3):805–820. doi: 10.1128/jvi.30.3.805-820.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachenheimer S. L., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus. VI. Polyadenylic acid sequences in viral messenger ribonucleic acid. J Virol. 1972 Oct;10(4):875–879. doi: 10.1128/jvi.10.4.875-879.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bartkoski M., Roizman B. RNA synthesis in cells infected with herpes simple virus. XIII. Differences in the methylation patterns of viral RNA during the reproductive cycle. J Virol. 1976 Dec;20(3):583–588. doi: 10.1128/jvi.20.3.583-588.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. B., Watson R. J., Wilkie N. M. Temporal regulation of herpes simplex virus type 1 transcription: location of transcripts on the viral genome. Cell. 1977 Sep;12(1):275–285. doi: 10.1016/0092-8674(77)90205-7. [DOI] [PubMed] [Google Scholar]
- Courtney R. J., Schaffer P. A., Powell K. L. Synthesis of virus-specific polypaptides by temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1976 Dec;75(2):306–318. doi: 10.1016/0042-6822(76)90030-1. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dunn A. R., Hassell J. A. A novel method to map transcripts: evidence for homology between an adenovirus mRNA and discrete multiple regions of the viral genome. Cell. 1977 Sep;12(1):23–36. doi: 10.1016/0092-8674(77)90182-9. [DOI] [PubMed] [Google Scholar]
- Grafstrom R. H., Alwine J. C., Steinhart W. L., Hill C. W., Hyman R. W. The terminal repetition of herpes simplex virus DNA. Virology. 1975 Sep;67(1):144–157. doi: 10.1016/0042-6822(75)90412-2. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Jacob R. J., Wadsworth S. C., Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C. A., Oliver S. G., Newman A. M., Holland L. E., McLaughlin C. S., Wagner E. K., Warner R. C. The molecular weight of yeast P1 double-stranded RNA. J Biol Chem. 1978 Nov 25;253(22):8332–8336. [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. C., Hayward G. S., Roizman B. Anatomy of herpes simplex virus DNA VII. alpha-RNA is homologous to noncontiguous sites in both the L and S components of viral DNA. J Virol. 1977 Jan;21(1):268–276. doi: 10.1128/jvi.21.1.268-276.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M., Roizman B. Regulation of herpesvirus macromolecular synthesis: nuclear retention of nontranslated viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4322–4326. doi: 10.1073/pnas.71.11.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland N. J., McDougall J. K. Biochemical transformation of mouse cells by fragments of herpes simplex virus DNA. Cell. 1977 May;11(1):233–241. doi: 10.1016/0092-8674(77)90334-8. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Gershowitz A., Stringer J. R., Holland L. E., Wagner E. K. 5'-Terminal and internal methylated nucleosides in herpes simplex virus type 1 mRNA. J Virol. 1977 Aug;23(2):234–239. doi: 10.1128/jvi.23.2.234-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes B. E., Stark G. R. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. doi: 10.1016/0092-8674(75)90105-1. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Davison A. J., Marsden H. S., Timbury M. C., Subak-Sharpe J. H., Wilkie N. M. Recombinants between herpes simplex virus types 1 and 2: analyses of genome structures and expression of immediate early polypeptides. J Virol. 1978 Nov;28(2):499–517. doi: 10.1128/jvi.28.2.499-517.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purifoy D. J., Lewis R. B., Powell K. L. Identification of the herpes simplex virus DNA polymerase gene. Nature. 1977 Oct 13;269(5629):621–623. doi: 10.1038/269621a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Silvertien S., Millette R., Jones P., Roizman B. RNA synthesis in cells infected with herpes simplex virus. XII. Sequence complexity and properties of RNA differing in extent of adenylation. J Virol. 1976 Jun;18(3):977–991. doi: 10.1128/jvi.18.3.977-991.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stringer J. R., Holland L. E., Swanstrom R. I., Pivo K., Wagner E. K. Quantitation of herpes simplex virus type 1 RNA in infected HeLa cells. J Virol. 1977 Mar;21(3):889–901. doi: 10.1128/jvi.21.3.889-901.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer J. R., Holland L. E., Wagner E. K. Mapping early transcripts of herpes simplex virus type 1 by electron microscopy. J Virol. 1978 Jul;27(1):56–73. doi: 10.1128/jvi.27.1.56-73.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland B. M., Rice M., Wagner E. K. Xeroderma pigmentosum cells contain low levels of photoreactivating enzyme. Proc Natl Acad Sci U S A. 1975 Jan;72(1):103–107. doi: 10.1073/pnas.72.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R. I., Pivo K., Wagner E. K. Restricted transcription of the herpes simplex virus genome occurring early after infection and in the presence of metabolic inhibitors. Virology. 1975 Jul;66(1):140–150. doi: 10.1016/0042-6822(75)90185-3. [DOI] [PubMed] [Google Scholar]
- Swanstrom R. I., Wagner E. K. Regulation of synthesis of herpes simplex type 1 virus mRNA during productive infection. Virology. 1974 Aug;60(2):522–533. doi: 10.1016/0042-6822(74)90346-8. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Wadsworth S., Jacob R. J., Roizman B. Anatomy of herpes simplex virus DNA. II. Size, composition, and arrangement of inverted terminal repetitions. J Virol. 1975 Jun;15(6):1487–1497. doi: 10.1128/jvi.15.6.1487-1497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B. RNA synthesis in cells infected with herpes simplex virus. II. Evidence that a class of viral mRNA is derived from a high molecular weight precursor synthesized in the nucleus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):626–633. doi: 10.1073/pnas.64.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Swanstrom R. I., Rice M., Howell L., Lane J. Variation in the molecular size of the DNA from closely related strains of type I herpes simplex virus. Biochim Biophys Acta. 1976 Jun 18;435(2):192–205. doi: 10.1016/0005-2787(76)90250-1. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Tewari K. K., Kolodner R., Warner R. C. The molecular size of the herpes simplex virus type 1 genome. Virology. 1974 Feb;57(2):436–447. doi: 10.1016/0042-6822(74)90183-4. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. Characterization of transcription-deficient temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1978 Dec;91(2):364–379. doi: 10.1016/0042-6822(78)90384-7. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Cortini R. Sequence arrangement in herpes simplex virus type 1 DNA: identification of terminal fragments in restriction endonuclease digests and evidence for inversions in redundant and unique sequences. J Virol. 1976 Oct;20(1):211–221. doi: 10.1128/jvi.20.1.211-221.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]