Abstract
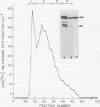
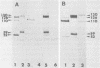
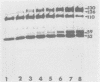
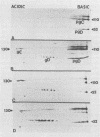
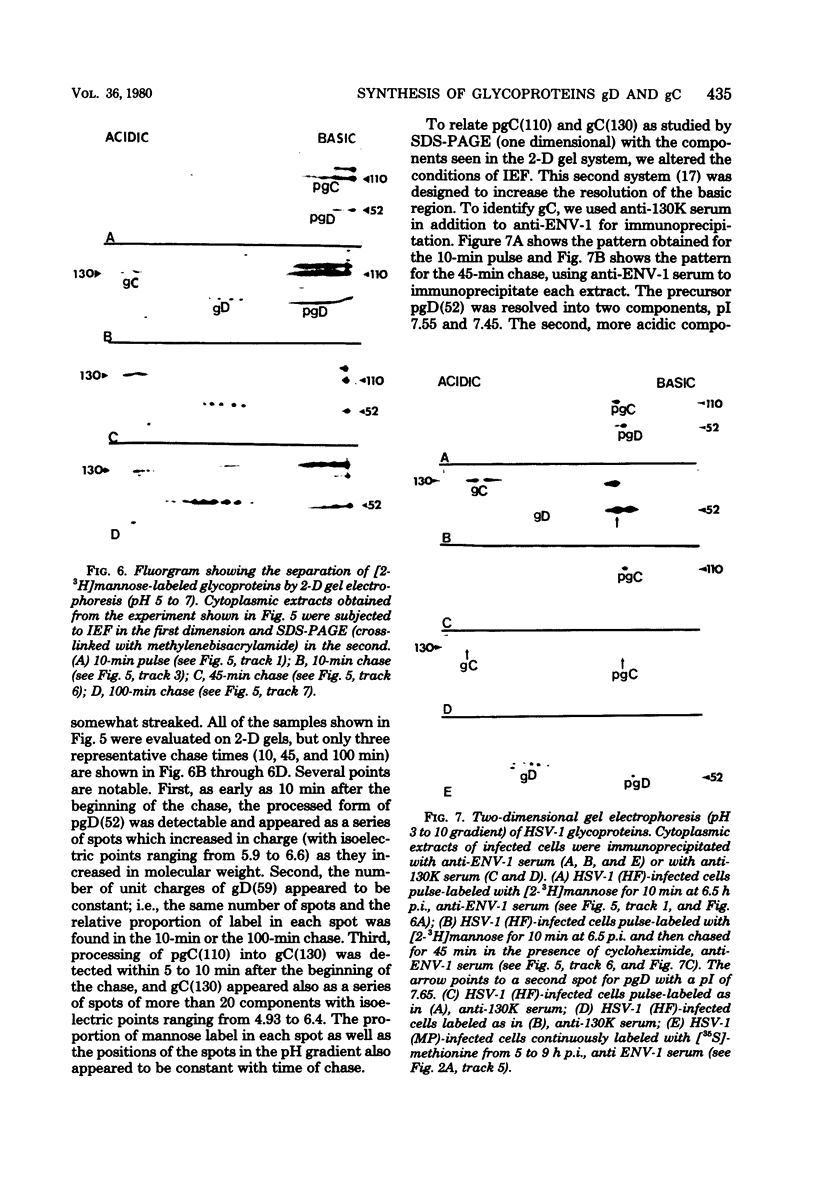
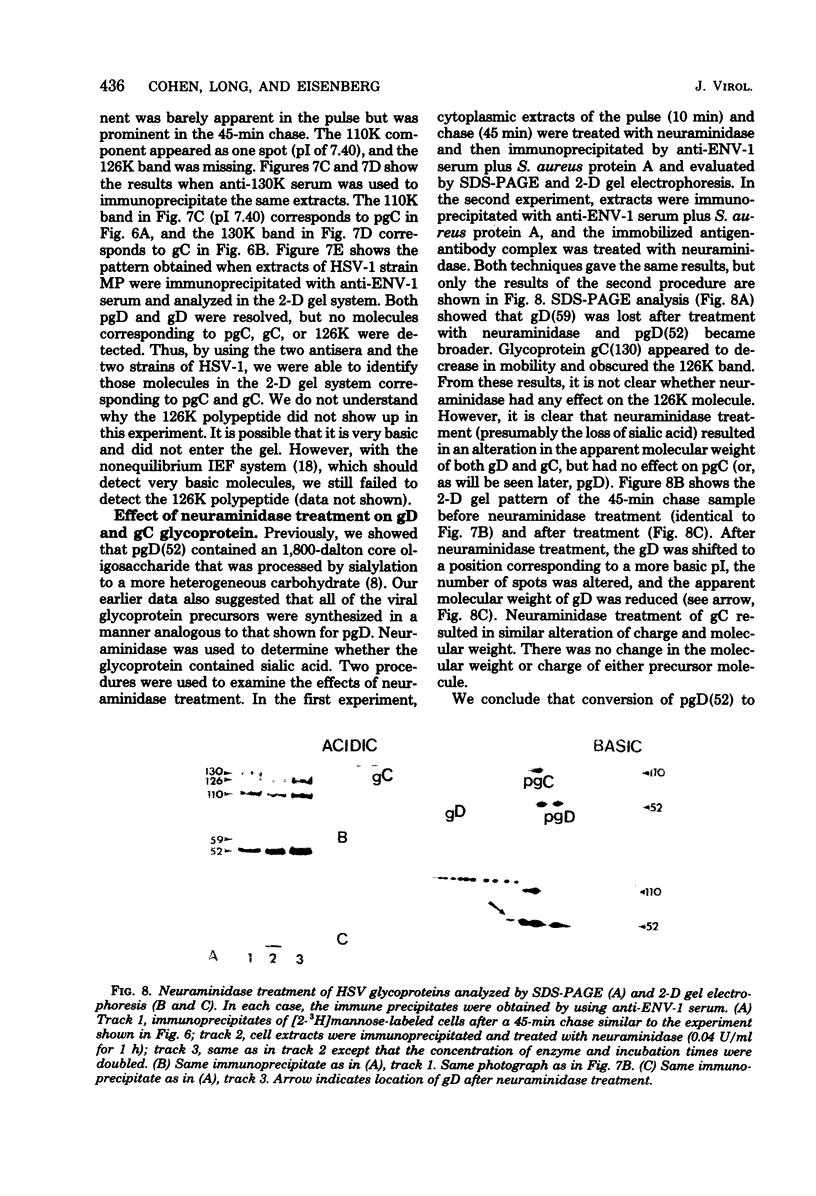
Herpes simplex virus type 1 (HSV-1) contains five glycoproteins, designated gA, gB, gC, gD, and gE. The present studies focused on the synthesis and processing of two of these, gC and gD. By using monoprecipitin antibody to gC, we demonstrated an antigenic and structural relationship between the precursor, pgC(110), and the product, gC(130). Tryptic peptide analysis showed that pgC and gC shared methionine peptides and that these molecules had the same fingerprint pattern as that of gC(130) extracted from the purified virion. These results suggested that post-translational processing of gC involved no major changes in methionine-containing tryptic peptides or in the cleavage sites required to generate those peptides. The syntheses of gC and gD were compared. We found that the glycoproteins were synthesized starting at different times in the infectious cycle; pgD was detected by 2 h postinfection, whereas pgC was first detected at 4 to 6 h postinfection. Both precursor molecules, pgC(110) and pgD(52), are basic glycopolypeptides, and in both cases processing involved changes in molecular weight and charge. These changes were detected by two-dimensional gel electrophoresis. Both glycoproteins exhibited heterogeneity, displayed as a series of spots (6 for gD and 15 to 20 for gC) of increasing negative charge and molecular weight. Neuraminidase treatment decreased the size, number, and acidic charge of the spots, suggesting that processing was due in part, but not entirely, to addition of sialic acid to pgD and pgC.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen G. H., Factor M. N., Ponce de Leon M. Inhibition of herpes simplex virus type 2 replication by thymidine. J Virol. 1974 Jul;14(1):20–25. doi: 10.1128/jvi.14.1.20-25.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Ponce de Leon M., Diggelmann H., Lawrence W. C., Vernon S. K., Eisenberg R. J. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J Virol. 1980 May;34(2):521–531. doi: 10.1128/jvi.34.2.521-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Ponce de Leon M., Nichols C. Isolation of a herpes simplex virus-specific antigenic fraction which stimulates the production of neutralizing antibody. J Virol. 1972 Nov;10(5):1021–1030. doi: 10.1128/jvi.10.5.1021-1030.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Vaughan R. K., Lawrence W. C. Deoxyribonucleic acid synthesis in synchronized mammalian KB cells infected with herpes simplex virus. J Virol. 1971 Jun;7(6):783–791. doi: 10.1128/jvi.7.6.783-791.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J., Powell K. L. Immunological and biochemical characterization of polypeptides induced by herpes simplex virus types 1 and 2. IARC Sci Publ. 1975;(11 Pt 1):63–73. [PubMed] [Google Scholar]
- Eisenberg R. J., Hydrean-Stern C., Cohen G. H. Structural analysis of precursor and product forms of type-common envelope glycoprotein D (CP-1 antigen) of herpes simplex virus type 1. J Virol. 1979 Sep;31(3):608–620. doi: 10.1128/jvi.31.3.608-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XIII. Glycosylation of viral polypeptides. J Virol. 1975 Nov;16(5):1308–1326. doi: 10.1128/jvi.16.5.1308-1326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Etchison J. R., Summers D. F. Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Feb;75(2):754–758. doi: 10.1073/pnas.75.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce de Leon M., Hessle H., Cohen G. H. Separation of Herpes simplex virus-induced antigens by Concanavalin A affinity chromatography. J Virol. 1973 Oct;12(4):766–774. doi: 10.1128/jvi.12.4.766-774.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr Demonstration of a surface difference between virions of two strains of herpes simplex virus. Virology. 1963 Feb;19:198–204. doi: 10.1016/0042-6822(63)90009-6. [DOI] [PubMed] [Google Scholar]
- Reed C. L., Cohen G. H., Rapp F. Detection of a virus-specific antigen on the surface of herpes simplex virus-transformed cells. J Virol. 1975 Mar;15(3):668–670. doi: 10.1128/jvi.15.3.668-670.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Sarmiento M., Manservigi R. The structural proteins and glycoproteins of herpesviruses: a review. IARC Sci Publ. 1978;(24 Pt 1):157–167. [PubMed] [Google Scholar]
- Tabas I., Schlesinger S., Kornfeld S. Processing of high mannose oligosaccharides to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J Biol Chem. 1978 Feb 10;253(3):716–722. [PubMed] [Google Scholar]
- Vestergaard B. F., Norrild B. Crossed immunoelectrophoresis of a herpes simplex virus type 1-specific antigen: immunological and biochemical characterization. J Infect Dis. 1978 Nov;138(5):639–643. doi: 10.1093/infdis/138.5.639. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]