Abstract
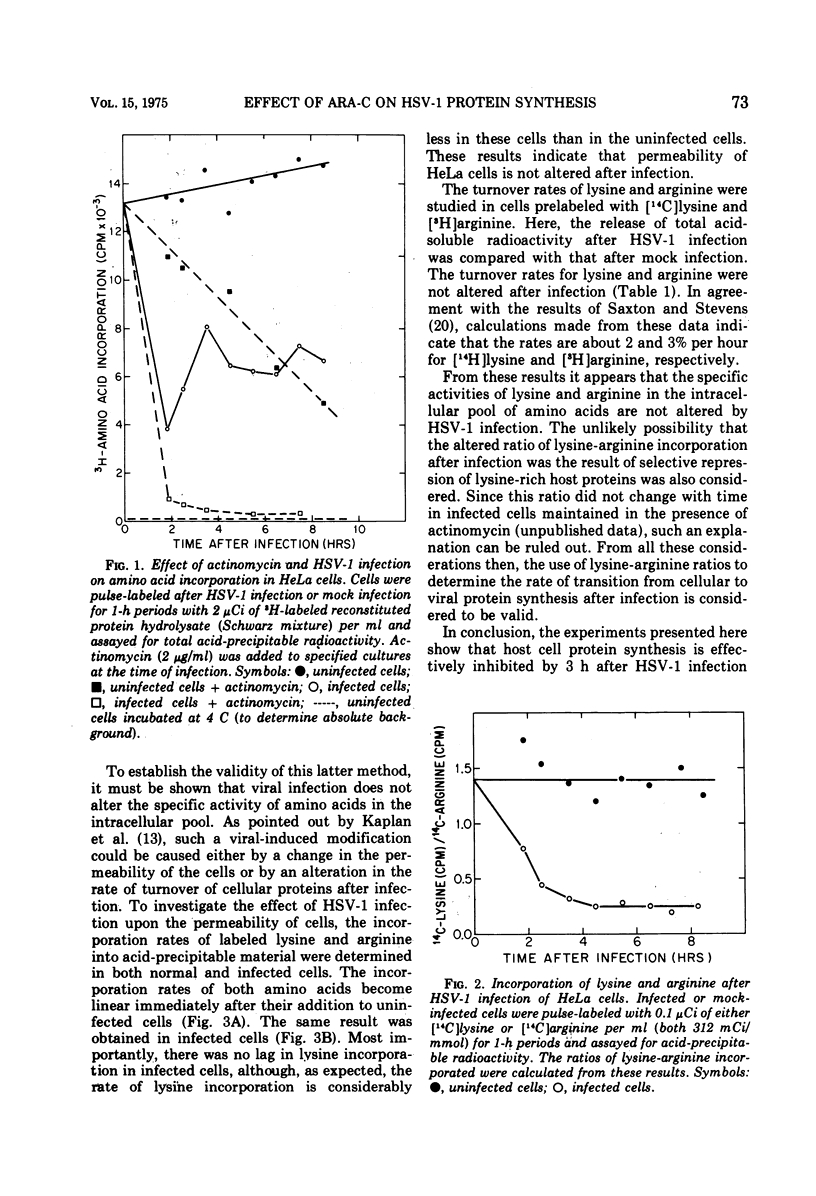
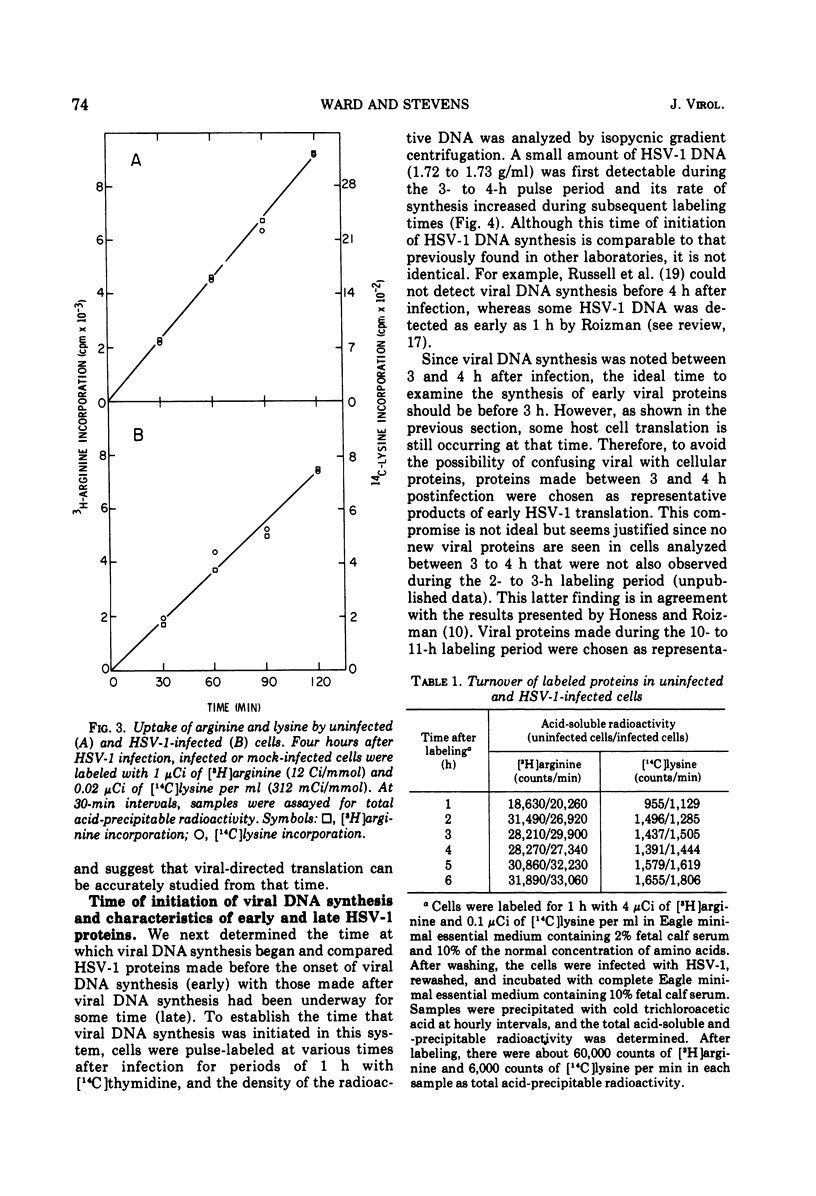
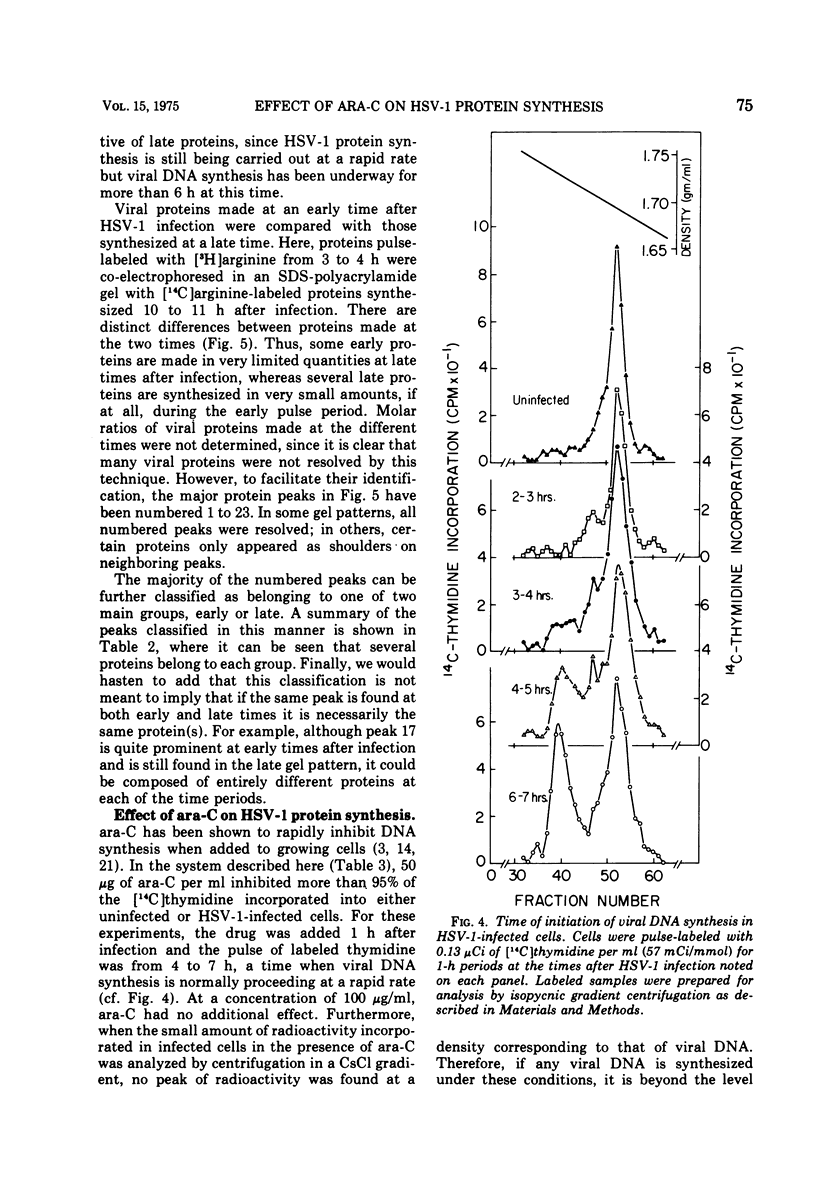
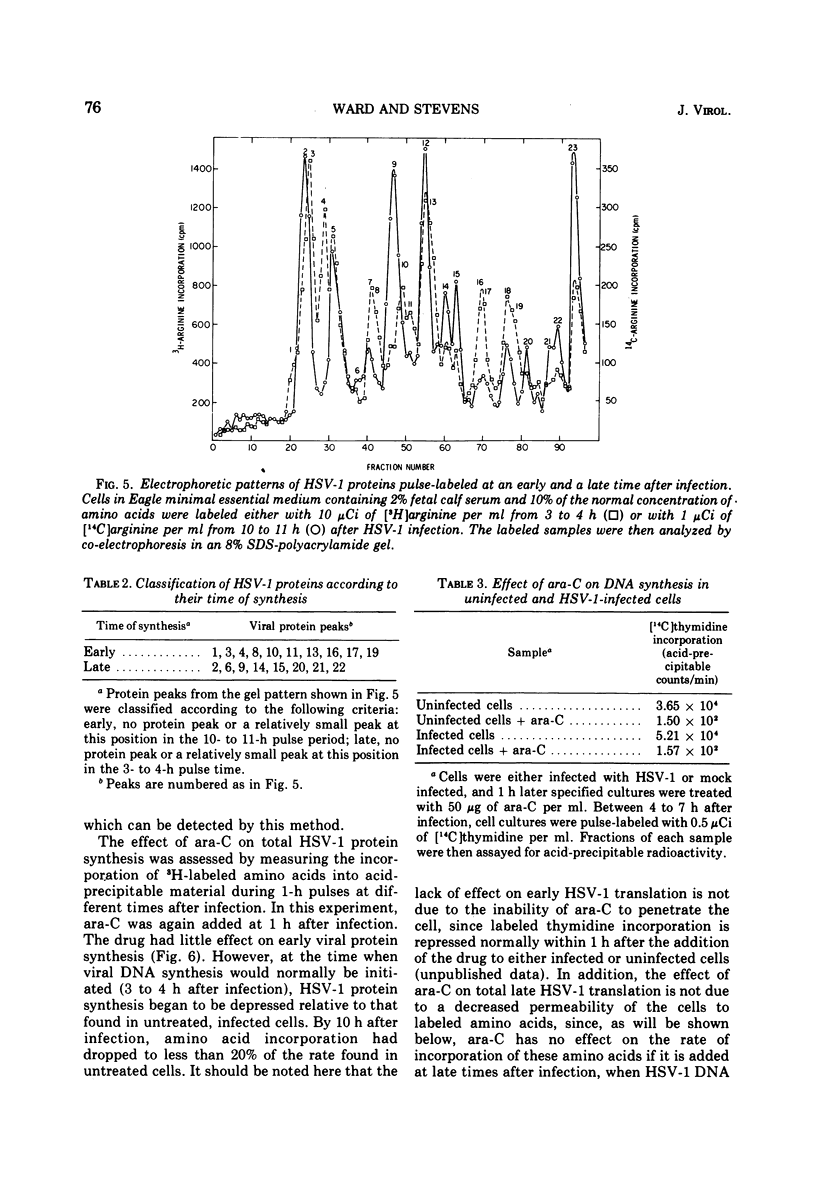
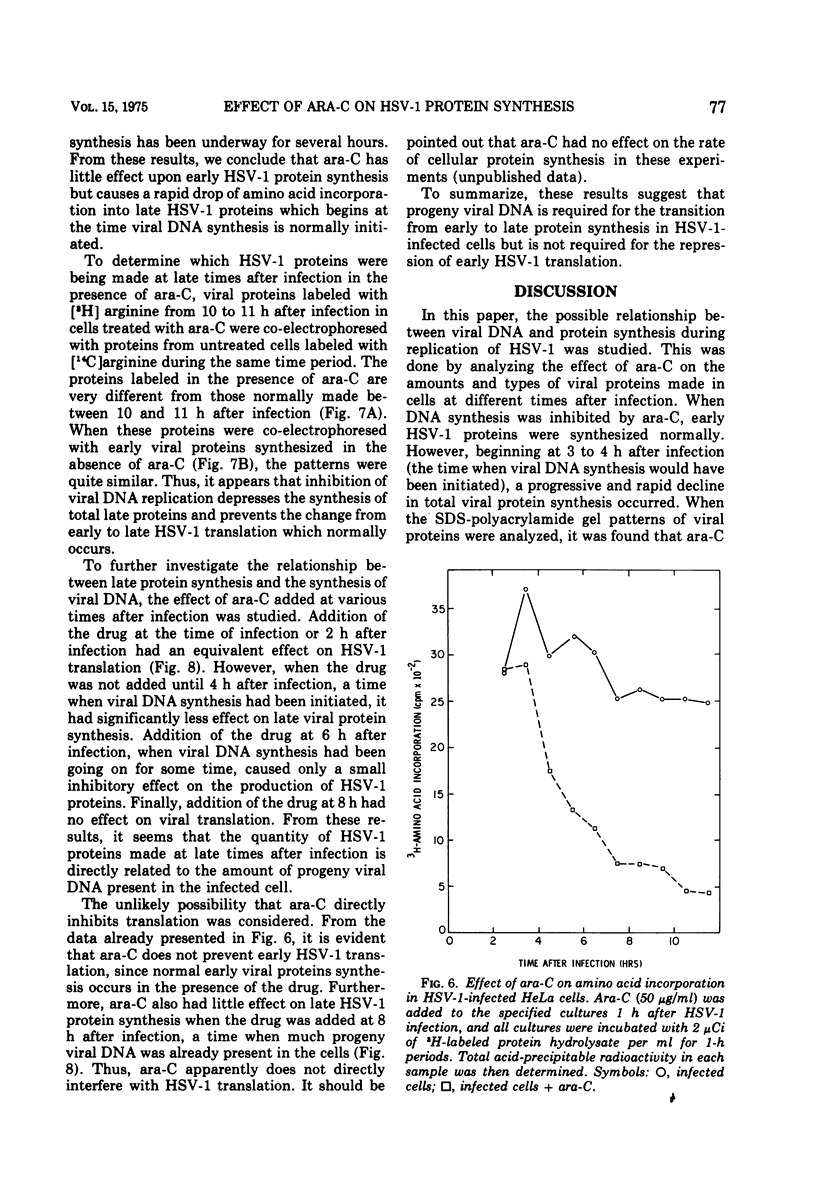
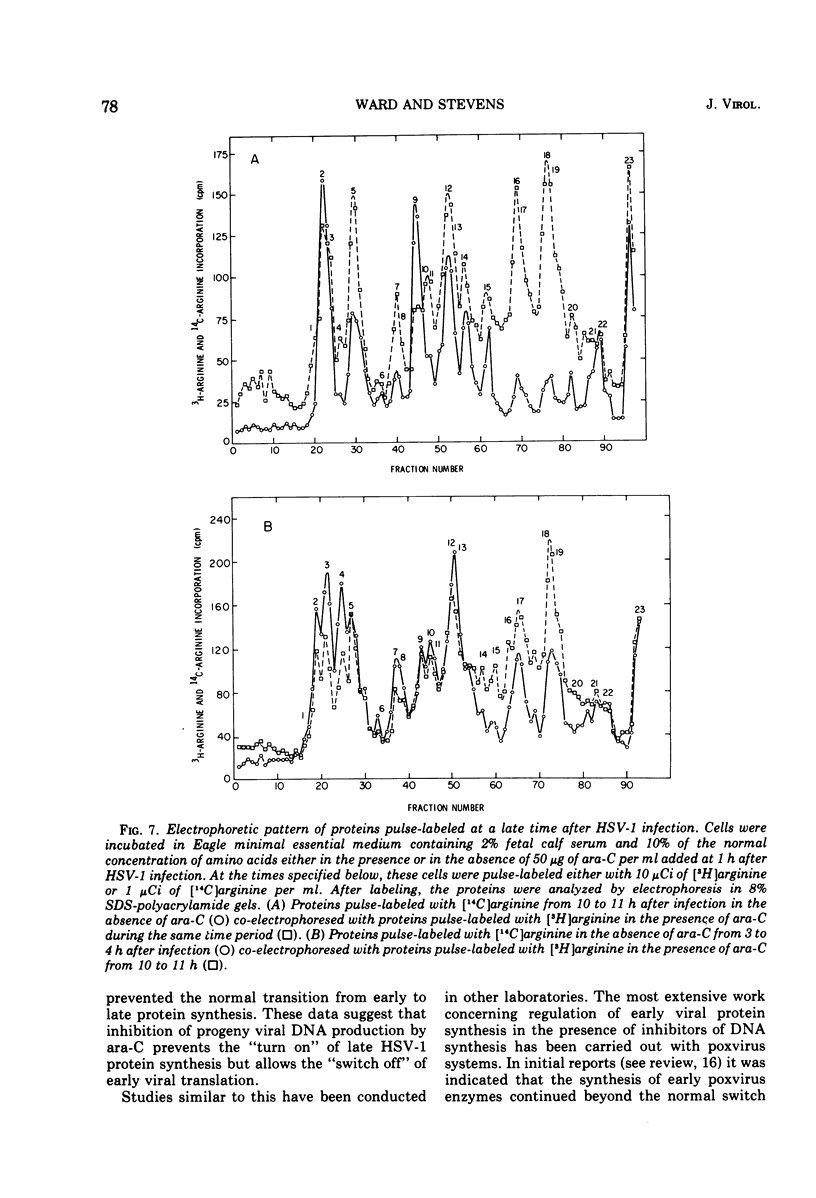
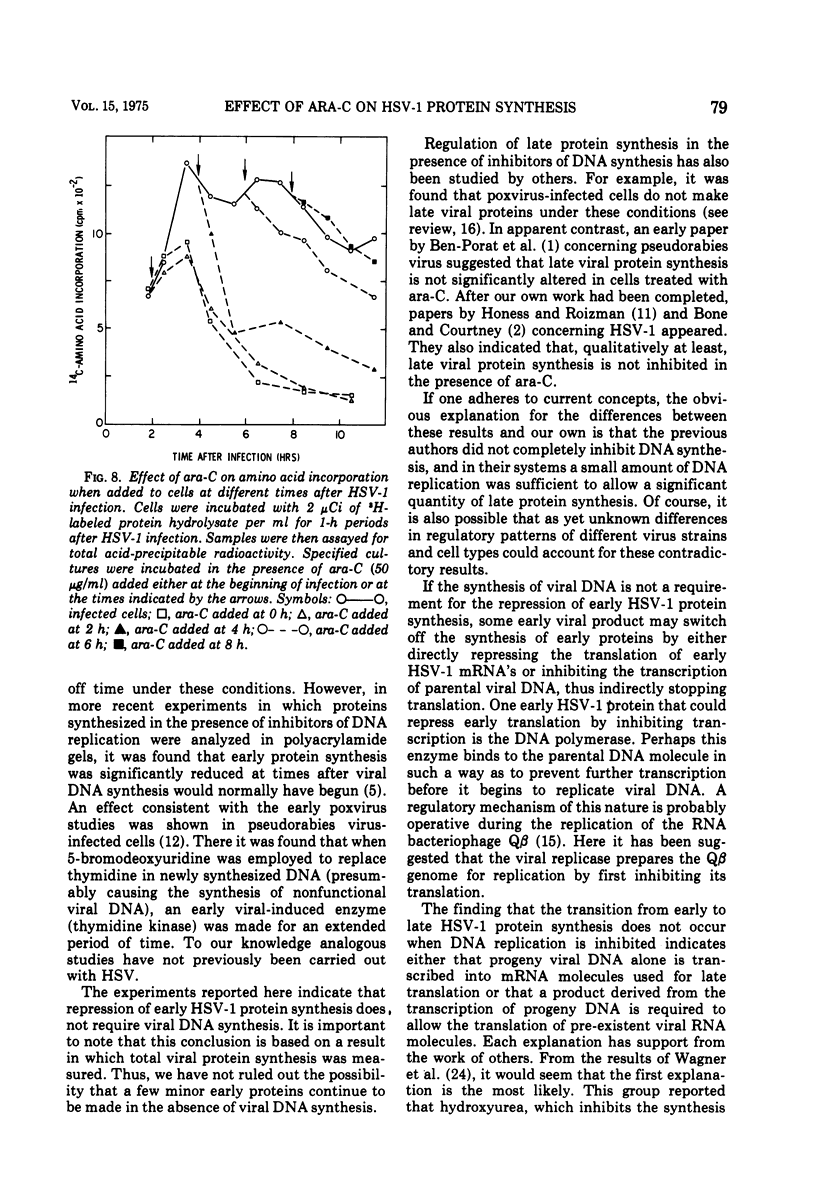
The relationship between viral DNA and protein synthesis during herpes simplex virus type 1 (HSV-1) replication in HeLa cells was examined. Treatment of infected cells with cytosine arabinoside (ara-C), which inhibited the synthesis of HSV-1 DNA beyond the level of detection, markedly affected the types and amounts of viral proteins made in the infected cell. Although early HSV-1 proteins were synthesized normally, there was a rapid decline in total viral protein synthesis beginning 3 to 4 h after infection. This is the time that viral DNA synthesis would normally have been initiated. ara-C also prevented the normal shift from early to late viral protein synthesis. Finally, it was shown that the effect of ara-C on late protein synthesis was dependent upon the time after infection that the drug was added. These results suggest that inhibition of progeny viral DNA synthesis by ara-C prevents the "turning on" of late HSV-1 protein synthesis but allows early translation to be "switched off."
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Shimono H., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. II. Flow of structural viral proteins from cytoplasm to nucleus. Virology. 1969 Jan;37(1):56–61. doi: 10.1016/0042-6822(69)90306-7. [DOI] [PubMed] [Google Scholar]
- Bone D. R., Courtney R. J. A temperature-sensitive mutant of herpes simplex virus type 1 defective in the synthesis of the major capsid polypeptide. J Gen Virol. 1974 Jul;24(1):17–27. doi: 10.1099/0022-1317-24-1-17. [DOI] [PubMed] [Google Scholar]
- CHU M. Y., FISCHER G. A. A proposed mechanism of action of 1-beta-D-arabinofuranosyl-cytosine as an inhibitor of the growth of leukemic cells. Biochem Pharmacol. 1962 Jun;11:423–430. doi: 10.1016/0006-2952(62)90225-3. [DOI] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban M., Metz D. H. Early virus protein synthesis in vaccinia virus-infected cells. J Gen Virol. 1973 May;19(2):201–206. doi: 10.1099/0022-1317-19-2-201. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus: controls of transcription and of RNA abundance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2654–2658. doi: 10.1073/pnas.69.9.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Silverstein S., Cassai E., Roizman B. RNA synthesis in cells infected with herpes simplex virus. VII. Control of transcription and of transcript abundancies of unique and common sequences of herpes simplex virus 1 and 2. J Virol. 1973 Jun;11(6):886–892. doi: 10.1128/jvi.11.6.886-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodheart C. R., Plummer G., Waner J. L. Density difference of DNA of human herpes simplex viruses, types I and II. Virology. 1968 Jul;35(3):473–475. doi: 10.1016/0042-6822(68)90225-0. [DOI] [PubMed] [Google Scholar]
- Graham B. J., Ludwig H., Bronson D. L., Benyesh-Melnick M., Biswal N. Physicochemical properties of the DNA of herpes viruses. Biochim Biophys Acta. 1972 Jan 18;259(1):13–23. doi: 10.1016/0005-2787(72)90469-8. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM J. H., EIDINOFF M. L. ACTION OF 1-BETA-D-ARABINOFURANOSYLCYTOSINE ON THE NUCLEIC ACID METABOLISM AND VIABILITY OF HELA CELLS. Cancer Res. 1965 Jun;25:698–702. [PubMed] [Google Scholar]
- Kamiya T., Ben-Porat T., Kaplan A. S. Control of certain aspects of the infective process by progeny viral DNA. Virology. 1965 Aug;26(4):577–589. doi: 10.1016/0042-6822(65)90320-x. [DOI] [PubMed] [Google Scholar]
- Kaplan A. S., Shimono H., Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus. 3. Relative amino acid content of various proteins formed after infection. Virology. 1970 Jan;40(1):90–101. doi: 10.1016/0042-6822(70)90382-x. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Weissmann C. Possible mechanism for transition of viral RNA from polysome to replication complex. Nat New Biol. 1971 May 12;231(19):42–46. doi: 10.1038/newbio231042a0. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. C., CRAWFORD L. V. PROPERTIES OF THE NUCLEIC ACIDS FROM SOME HERPES GROUP VIRUSES. Virology. 1964 Feb;22:288–292. doi: 10.1016/0042-6822(64)90017-0. [DOI] [PubMed] [Google Scholar]
- Saxton R. E., Stevens J. G. Restriction of herpes simplex virus replication by poliovirus: a selective inhibition of viral translation. Virology. 1972 Apr;48(1):207–220. doi: 10.1016/0042-6822(72)90128-6. [DOI] [PubMed] [Google Scholar]
- Silagi S. Metabolism of 1-beta-D-arabinofuranosylcytosine in L cells. Cancer Res. 1965 Oct;25(9):1446–1453. [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Swanstrom R. I., Stafford M. G. Transcription of the herpes simplex virus genome in human cells. J Virol. 1972 Oct;10(4):675–682. doi: 10.1128/jvi.10.4.675-682.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


